Abstract
Phosphorus (P) is one of the essential macronutrients for crops. It is present in soil in two forms: soluble and insoluble. However, plants cannot absorb the insoluble forms, including Al-P, Fe-P, and Ca-P; thus, the phosphorus use efficiency is reduced. Therefore, the biological approaches should focus more on sustainable agriculture to overcome this constraint. This article cites publications relating to the biological P solubilizer group of bacteria, which have a highly potential adaptation to many conditions in soils. Among the biological approaches, purple nonsulfur bacteria (PNSB) are a potent group of bacteria according to their adaptability in acidic, saline, and toxic conditions based on their mechanisms in producing exopolymeric substances and siderophores under such adverse environments like acid-sulfate and saline soils. PNSB can solubilize P in soil to have more P availability for soil microbes and plants. This particular group of bacteria has been widely applied in liquid and solid forms from agricultural waste to promote plant growth under submerged conditions. Moreover, this article summarized the P-solubilizing mechanisms of P-solubilizing bacteria and introduced future research perspectives on patterns of PNSB in aspects of nutrient-providing potency, plant growth-promoting capability, and biological control capacity. However, the specific mechanisms of P solubilization by PNSB have not been well documented since the P-solubilizing mechanisms have been investigated on general P-solubilizing bacteria. Thus, specific pathways and metabolites relating to the P-solubilizing PNSB should be investigated, and attention should be addressed to them soon.
1 Introduction
More and more crops are suffering from nutrient limitation to their productivity in soils, and phosphorus (P) is one of the most critical limiting nutrients [1]. Consequently, farmers tend to use more P chemical fertilizer to maintain crop yield as expected. However, its use may become excessive [2,3]. Also, despite their excessive intake, plants take up only 10–20% of the P fertilizer applied to soil [4] because P is precipitated and absorbed by minerals, for example, Al, Fe, and Ca [5]. Overusing P chemical fertilizer can lead to soil acidification, destruction of food web systems, water contamination, and decreased soil fertility [6,7,8,9]. Moreover, the P chemical fertilizer types are considered one of the highest crop input costs [10,11]. That is why biological approaches have been conducted to find a long-term efficient way to increase P use efficiency in agriculture. Manure has been applied to reduce the amount of P chemical fertilizer in crop cultivation, and the results are positive; for example, replacing more than 50% of chemical fertilizer with manure improves soil fertility and P use efficiency [12]. However, microbial applications are more potent because the microbes can make use of insoluble P compounds existing in the soil [13] rather than supplying more P from organic sources. For instance, in the study by Emami et al. [14], rhizospheric and endophytic bacteria enhanced P use efficiency in wheat. The most well-known bacterial genera are Pseudomonas, Bacillus, Rhizobium, and Enterobacter [15]. Among them, using purple nonsulfur bacteria (PNSB) is a promising and suitable approach for soils in the Mekong Delta of Vietnam. This is because apart from solubilizing P, the PNSB can adapt to a broad range of conditions; that is, they can grow well under anaerobic light aerobic dark cycles [16], acidic and saline conditions [17,18], and contaminated environments [19]. The PNSB can also fix nitrogen and produce plant growth-promoting compounds, e.g., indole 3-acetic acid [20], 5-aminolevulinic acid (ALA) [21], and exopolymeric substances (EPS) [22]. Recently, there has been an increase in studies investigating the roles of P-solubilizing PNSB in soil health and crop yield [23,24,25,26]. Still, the ways in which insoluble P compounds are solubilized and how PNSB are used to function in agriculture have not been well summarized. Ultimately, in this article, the mechanisms of solubilizing P from insoluble fractions in soil by phosphorus-solubilizing bacteria (PSB) have been clarified and reviewed along with the recent achievements in studying P-solubilizing PNSB. Moreover, the current P status is also mentioned, and possible ways to apply PNSB to crops are also discussed.
2 Applications of PSB to achieve sustainable agriculture
2.1 Importance of phosphorus nutrients to the crops
There is no doubt that P is one of the most essential nutrients for crops because it is a part of many biological molecules, such as enzymes and nucleic acids. It has been investigated for nearly 200 years to determine its relationship between the yield of crops [27]. P is involved in many processes within a plant, such as the development of roots and stalks, formation of flowers and seeds, strength of stem, maturity, N-fixation processes, and fruit quality [28]. However, the cycle of P is different from other essential nutrients, such as nitrogen (N) and carbon (C), since it does not go through gaseous forms under the temperature and pressure of the Earth [29]. Additionally, inorganic P in soil is typically immobilized by many forms of soil series, for example, Fe, Ca, and Al, resulting in insoluble P compounds [30]. That is why P is the most immobilized element compared to other macronutrients [28]. Therefore, P use efficiency is poor. In the study by Johnston and Poulton [27], crop yield and P availability are positively correlated, from which a threshold of P fertilizer required can be found by the analytical method of Olsen. If soils possess available P near a certain critical level, P use by plants is effective; that is, P chemical fertilizer applied is equal to the amount of P taken up by crops, thereby keeping soil P near the critical level. This will not only make crop yield and P applied efficiency optimal but also inhibit P amount leaking to the environment [27].
2.2 Disadvantages of chemical fertilizers
Although inorganic P is extremely important to plants, it comes from nonrenewable resources [31]. The increase in food demands led to higher production of P chemical fertilizers [32]. There are many sources of P chemical fertilizer with different P2O5 contents. It can be divided into three main types of P chemical fertilizer: (1) water-soluble, including superphosphate (16–20% P2O5), monoammonium phosphate (61% P2O5), and diammonium phosphate (46% P2O5); (2) insoluble, including apatite (rock phosphate, 20–30% P2O5) and bone meal (18–20% P2O5); and (3) citrate soluble, including dicalcium phosphate (35–40% P2O5), and basic slag (3–5% P2O5) [33]. In most acidic soils like acid-sulfate soils (high amount of H+), P is precipitated with Al and Fe oxides and, thereby, becomes insoluble for plants to uptake. The P chemical fertilizer is considered to be an easy solution to improve the availability of soil P for plants to overcome the limitation of P in soils not being available to plants [34]. However, P fertilizer applications may cause severe damage to surrounding environments, such as acidification and hardening of the soil and soil fertility reduction [35]. Moreover, manure is another additional source of organic P, which helps improve soil fertility, especially available P content, and crop yield [36,37,38,39].
2.3 Status of phosphorus nutrients in the soil
Most soils have higher total P content than other macronutrients, such as N and potassium (K) [31]. However, 80% of P is precipitated and unavailable for plants to uptake [40]. The unavailable P portion in the soil is usually organic and inorganic forms (organic P and inorganic P), whose ratio is different throughout the soil types [41]. Organic P interacts with soil particles weakly, but the inorganic one remains in more stable forms [42]. The P applied to the soil in the form of chemical fertilizer is usually deficient in plants and stored in the soil as unavailable forms of P because of the adsorption, precipitation, and immobilization processes [43]. For the adsorption, available P from chemical fertilizers applied to the soil surface alters other anions, whose affinity is worse in soil particles and gets adsorbed [44,45]. Although some P desorption processes happen in soil, the desorption rates are much slower than others [46]. Precipitation occurs due to metallic cations within the soil, i.e., P is bound to Fe, Ca, and Al [47]. Common P minerals are determined to be the following: strengite (FePO4·2H2O) and variscite (AlPO4·2H2O) in acidic soils; and tricalcium phosphate [Ca3(PO4)2], dicalcium phosphate (CaHPO4), dicalcium phosphate dihydrate (CaHPO4·H2O), fluorapatite [Ca5(PO4)3F], hydroxyapatite [Ca5(PO4)3OH], and octacalcium phosphate [Ca4H(PO4)3·2–5H2O] in neutral and calcareous soils [48]. Therefore, after P is fertilized chemically into the soil, P adsorption and precipitation happen until reaching the equilibrium [44]. Moreover, in some soils, these processes can account for 80% of the total P amount in soil [49]. Although the primary mechanism of P solubilization in soil is low soil pH [28], mechanisms to solubilize organic P and inorganic P fractions in soil are found differently.
2.3.1 Organic P
Organic P accounts for 20% of total P in soil [50]. It remains in three types of bonds in soil, including phosphate esters, phosphatases, and phosphoric acid anhydrides [2]. Insoluble organic P compounds have to go through mineralization processes to make available P for plants [30]. These processes are facilitated by phosphatases and phytases secreted by soil microorganisms [51,52]. Phytase is known to be the primary enzyme responsible for releasing the organic P in soil [53]. The half-life of the extracellular phosphatases secreted by soil microbes is usually short in soil because they can be inactivated by metal inhibitors, adsorption, proteolysis, pH, and ionic strength shifts [54]. Significantly, PSB have been reported to be able to enhance the activity of soil phosphatase. A similar reveal has also been found in the study by Khuong et al. [55], where the soil phosphatase activity was found to increase when PNSB strains of Rhodopseudomonas palustris were applied into the soil, in comparison to the control treatment without inoculation. In other words, the density of PNSB applied was positively correlated with the phosphatase enzyme activity; that is, these PNSB can produce phosphatase. Because plants can use only inorganic P to mobilize P from organic P compounds, a two-step process is required, consisting of P being released from precipitating and adsorbing positions and then mineralized by enzymes [56,57]. On the contrary, pH is also found to drive the P-solubilizing genes and microbial community composition [58]. When the culture pH dropped to 4.55, soluble P and the activity of acid phosphatase produced by Ochrobactrum sp. J023 were valued at 161.29 mg L−1 and 61.98 U mL−1, respectively [59].
2.3.2 Inorganic P
Popular forms of insoluble inorganic P are calcium phosphate [Ca3(PO4)2], aluminum phosphate (AlPO4), ferrous phosphate (FePO4), zinc phosphate [(Zn3(PO4)2], and manganese phosphate [(Mn3(PO4)2], which can be dissolved into soluble P under the contribution of microorganisms in the soil [30,60,61,62,63,64]. Although there have been many theories involved in microorganisms, the secretion of low molecular mass organic acids (OAs) has been considered a well-studied and accepted one as a principal mechanism of P-solubilization and those OAs have been studied for their identification and roles in solubilizing processes [65,66,67]. OAs are low molecular weight compounds and can solubilize P by chelating the phosphorus-bound cation with OAs’ hydroxyl and carboxyl groups to release the linkage between the P and the cations and simultaneously lower the rhizospheric pH [68]. Those acids usually are oxalic, lactic, citric, succinic, acetic, and formic acids [69,70]. The OAs can chelate metal ions to release P from the insoluble sources [71] or reduce soil pH to result in proton substitution for Ca2+ [72]. In the study by Maliha et al. [66], the production of OAs by the bacteria and fungi to solubilize P was roughly 2.4747 and 1.835 g L−1, respectively, along with the drop from pH 7.0 to below pH 3.0. A species of fungi, Penicillium oxalicum, was found to be able to solubilize P and lower medium pH at the same time, and the production of OAs by this fungus was lactic acid (0.054 mmol L−1), acetic acid (0.054 mmol L−1), oxalic acid (0.064 mmol L−1) with pH from 7.0 to 2.0 [70].
2.4 Biological P solubilization as an effective alternative
P-solubilizing microorganisms take part in remedying P deficiency for crops in soil. They can solubilize P for themselves and a vast amount of soluble phosphate to the environment in a quantity that exceeds the microbes’ P demand [73]. Some bacteria are found to be produced by OAs and phosphatases [13], which are key mechanisms of P solubilization and mineralization, respectively, and were isolated from peat soil [74]. Thus, this excess production of soluble P can be a source of the P nutrient for plants. Either individual or mixed application of P-solubilizing microbes (optionally with other beneficial microbes, e.g., bacteria) enhanced yield and P uptake of crops [28]. They appear in all kinds of soil, but their diversity and quantity differ from soil types and climates [73]. Moreover, higher concentrations of P solubilized by microbes were found in solid environments rather than liquid ones. For instance, in the study by Anu and Kundu [75], in chickpeas, 76 strains of PSB were isolated with P-solubilizing efficiencies ranging from 6 to 118% for the solid medium, while in the broth, the number was from 22 to 248 μg/mL. Moreover, the PSB strains obtained from the rhizosphere of tomatoes in the study by Kouam et al. [76] solubilized P from 277.44 to 1929.29 μg/mL in a liquid medium. Additionally, Acinetobacter pittii gp-1 solubilized P roughly from 7.91 to 250.77 μg/mL in liquid medium and from 3.80 to 7.11 μg/mL in solid medium [77]. Furthermore, in the study by Amri et al. [78], a PSB, Pseudomonas fluorescens, isolated from sandy soils showed a great P solubilization, roughly from 535.70 to 618.57 μg/mL. Additionally, from phosphate solid sludge, PSB strains of Pseudomonas, Serratia, Pantoea, and Enterobacter showed a high P-solubilizing capacity from 101.91 and 174.33 μg/mL [79]. Therefore, applying PSB into the soil helps reduce the chemical P fertilizer application but still supports crop growth and yield performance [73]. By improving crop productivity, soil health also benefits from P [80] in an environmentally friendly, inexpensive, and effective way [81].
3 Phosphate-solubilizing bacteria
3.1 Diversity of phosphate-solubilizing bacteria and their mechanisms
Bacteria and fungi account for the majority of P-solubilizing microorganisms. Of these microorganisms, bacteria, including Escherichia freundii, Bacillus subtilis, Pseudomonas spp., Arthrobacter spp., Bacillus spp., B. firmus B-7650, P. radicum, Enterobacter agglomerans, Bacillus amyloliquefaciens, Bacillus licheniformis, Penibacillus macerans, Xanthobacter agilis, Enterobacter aerogenes, Enterobacter intermedium, and P. fluorescens, make a more significant contribution than fungi [28]. Their habitat is mainly in the rhizosphere. PSB, for example, Bacillus, Burkholderia, Azospirillum, Erwinia, Pseudomonas, Rhizobium, and Serratia, are known to be able to transform insoluble P forms into soluble forms that plants may assimilate [82,83]. They play an essential role in the cycle of P, while they mineralize organic P, solubilize inorganic P, and store P in their biomass [84,85]. Some bacteria are salt-tolerant and capable of solubilizing P. Thereby, they can maintain P-solubilization in saline soils [73]. In the study conducted by Baliah et al. [86], some novel PSB were identified as Bacillus megaterium, Pseudomonas putida, and P. fluorescens. As mentioned above, there are two ways to solubilize P compounds in soil, regardless of whether they are organic or inorganic forms. For inorganic sources, the fundamental principle is to lower the pH of the soil by causing soil bacteria to produce low molecular weight OAs [73]. These acids can either release P as the mechanism of anion exchange or chelate with metal ions, such as Fe, Al, and Ca, which bind to P [73]. In the study by Behera et al. [87], a PSB strain PSB-37, whose identity was Serratia sp., was isolated from mangrove soil. This strain possessed the highest P-solubilizing activity at 44.84 μg/mL. Concurrently, the pH of the culture medium dropped from 7.00 to 3.15. Moreover, many OAs are found within the culture during the inoculation time, for example, malic acid 237.0 mg/L), lactic acid (599.5 mg/L), and acetic acid (5.0 mg/L). Moreover, the use of efficient OAs and the production of inorganic acids (H2CO3), exopolysaccharides, and siderophores contribute to P solubilization. Conversely, for organic P compounds, the term “mineralization” is used, i.e., activities of enzymes of phosphatases and phytase carry out the hydrolysis of ester or anhydride bonds of phosphate compounds and finally release soluble P into the soil [73]. Genes responsible for P cycles in soil have been analyzed in the study by Liang et al. [85]. In this research, 36 genes accountable for P cycles are found and divided into three functions: solubilization (mineralizing organic P and solubilizing inorganic P), transporter, and regulatory genes. Among these, gcd, phoD, and phoA account for 70% of abundance, and gcd can be considered the most important in the P solubilization process. However, the P solubilization of the PSB sometimes causes a decrease in pH due to the secretion of OAs by the bacteria [88]. Moreover, this is not suitable for farming on acidic soils, whose pH is already low. In particular, Vietnamese soils are suffering from both acidification and salinization. Nevertheless, under the P solubilization by the PNSB, the soil pH did not decrease in the studies by Huu et al. [23] and Khuong et al. [89]. Moreover, the PNSB can be active in aerobic and anaerobic conditions [90]. This makes the PNSB suitable for the Mekong River Delta’s wet–dry cycles [91].
3.2 PNSB
3.2.1 P-solubilizing PNSB
Among the PSB, PNSB are capable of interacting with plants [92], thus enhancing plant growth by solubilizing P [89,93] and secreting phytohormones [94]. As in the PSB group, PNSB may have similar P-solubilizing mechanisms. However, studies on the P-solubilizing mechanisms of PNSB are still rare. For example, in the study by Khuong et al. [95], siderophores are found to be a P-solubilizing metabolite produced by PNSB. Furthermore, Lo et al. [96] found some genes coding for phosphatase, lyase CP, phosphatase inositol-phosphate, and OAs, which are related to the P solubilization processes in the genomes of PNSB species. Therefore, further studies should take place to gain the molecular level of knowledge about the P-solubilization of this group of bacteria. Moreover, the PNSB can also adapt to a variety of field conditions, such as acidic [95], saline [17], acidic saline [89], and contaminated conditions [19]. This makes the PNSB group different from the PSB and promising in applying to different types of soils, especially soils being transformed by climate change and overfertilization [97,98]. The major groups of PNSB in agriculture are Rhodopseudomonas spp. and Rhodobacter spp. [99]. Their natural habitats are humid soils, wastewater ponds, lagoons, lakes, sediments, high-salt systems, wetland ecosystems, and marine ecosystems [100,101,102]. Most PNSB can live under aerobic dark or anaerobic light conditions [103]. The PNSB can live both as an autotroph and as a chemotroph based on the energy source. Moreover, the PNSB can also produce substances to reduce the adverse effects of the environment, such as ALA against salinity [104], EPS against acidity and salinity [105], and siderophores against heavy metals [106]. This makes them promising for being applied in soil in the Mekong River Delta, Vietnam, where soil and crops are usually submerged. Moreover, ALA and siderophores are believed to involve mechanisms to solubilize P [107]. Some applications of PNSB have been made in agriculture, and the outcomes are very interesting. Specifically, in rice and sugar leaf, yield rises by 29 and 69.2%, respectively [92,108]. In addition, PNSB are also able to synthesize bioactive compounds, such as bioherbicides, antiviral substances, and antimicrobial compounds for plant protection from pathogenic microbes [94]. They can also synthesize riboflavin, iron carriers, ALA, EPS, and bacterial acyl-high serine lactone for stimulating plant resistance to diseases [109]. In addition, the P solubilization usually relates to the pH reduction [73]. This is not appropriate for use in acidic soils, while some applications of PNSB to solubilize P increase soil pH [23,24,93]. Thus, the PNSB are suitable candidates to solubilize P in acidic soils. Some PNSB species determined to be able to solubilize P have been identified as R. palustris [110,111], Luteovulum sphaeroides [93], and Rhodospirillum rubrum [112]. Figure 1 shows how these bacteria improve plant growth. In particular, the P-solubilizing PNSB increases P availability by P solubilization and produces plant growth-promoting substances, leading to better root development and greater leaf area, that is, better photosynthesis capacity and a better crop yield [20].
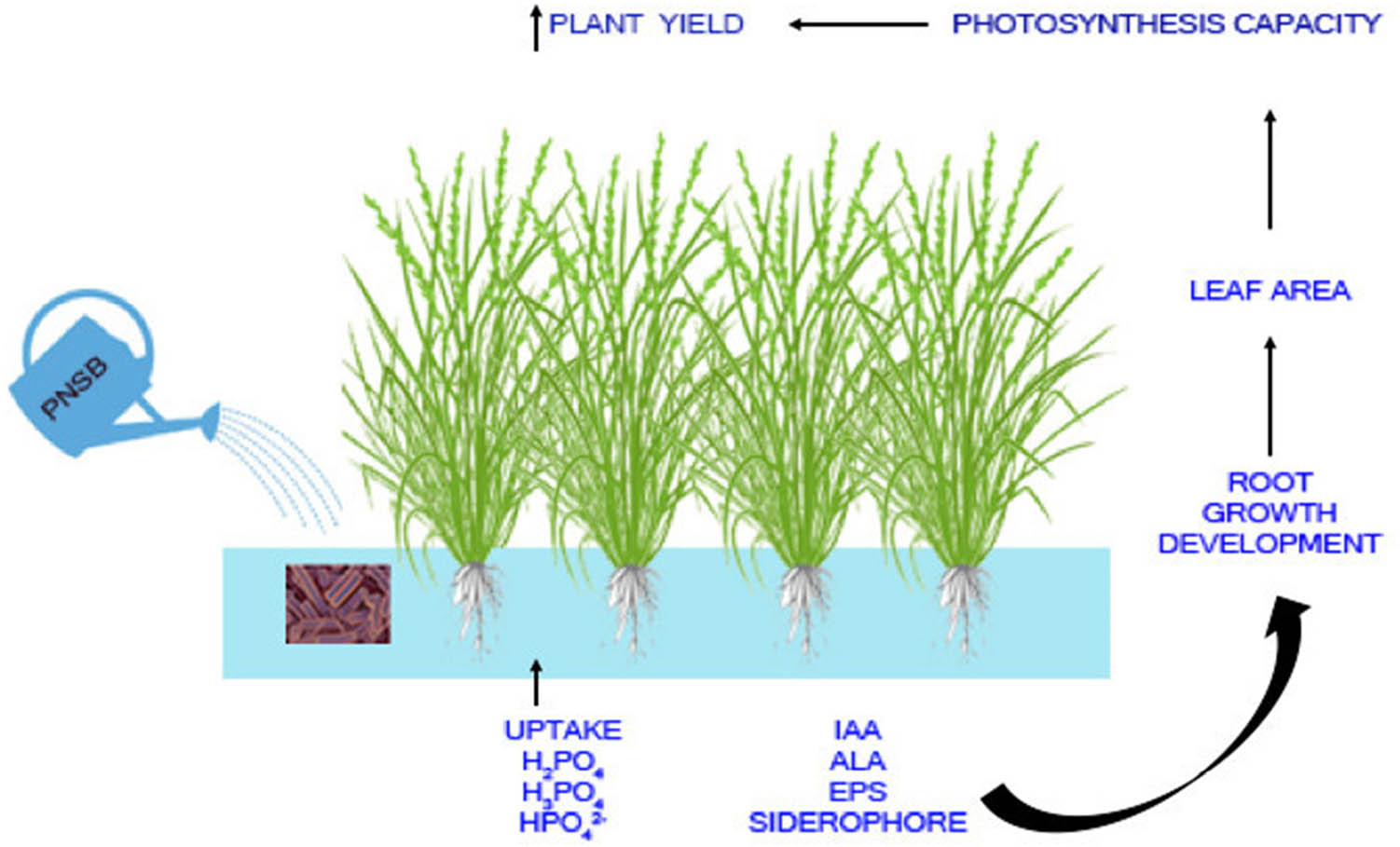
A summary of crop yield improvement by P-solubilizing PNSB. Source: Prepared by Authors.
3.2.2 Recent applications of PNSB: Mixed and single-use
Forming spores is an essential property of PNSB as biofertilizers. Other P-solubilizing microbes that do not produce spores in their life cycle are more vulnerable when applied on the field [113,114]. Moreover, their inoculants need to be in an appropriate formula; if not, the bacteria may be damaged, short in half-life, and unable to survive under adverse conditions [115]. Some PNSB strains have been used as biofertilizers for different types of crops, as shown in Table 1.
Types, origins, targets, and functions of recent PNSB biofertilizers
| PNSB biofertilizer | Form | Origin | Target | Function | Chemical fertilizer replacement | Reference |
|---|---|---|---|---|---|---|
| Rhodopseudomonas palustris spp. | N/A | Peat swamp forest | Rice in acidic soil | Biocontrol, production of PGPS, N2-fixation, and P-solubilization | N/A | [111] |
| Rhodopseudomonas palustris TK103, PP803, and P1 (6.7–6.8 log CFU mL−1) | Solid | Rubber sheet wastewater and saline paddy soils | Rice in saline soils | Production of PGPS reduces methane emissions | N/A | [93] |
| Rhodopseudomonas palustris VNW64, VNS89, TLS06 and VNS02 (108 cells g−1) | Solid | Acidic paddy soils | Sesame in salt-affected soil | N2-fixation and P-solubilization | 50% N and 50% P | [116] |
| Rhodopseudomonas palustris TLS06, VNW02, VNW64, and VNS89 (108 CFU mL−1) | Liquid | Acidic paddy soils | Rice in acid sulfate soil | Al3+ and Fe2+ reduction, N2-fixation, and P-solubilization | 25% N and 50% P | [117] |
| Rhodobacter sphaeroides W48 and W42 (108 cells mL−1) | Liquid | Acid sulfate soil | Pineapple in acid sulfate soil | P-solubilization | 25% P | [23] |
| Rhodopseudomonas palustris ISP-1 (108 CFU mL−1) | Liquid | Peanut fields | Peanut | P-solubilization and organic P-mineralization | N/A | [25] |
| Rhodopseudomonas palustris VNW64, VNS89, TLS06, and VNW02 (108 cells mL−1) | Liquid | Acidic soils | Canary melon in alluvial soil | P-solubilization | 25% P | [24] |
| Rhodopseudomonas palustris YSC3, YSC4, and PS3 (4.0 × 106 CFU g−1 soil) | Liquid | Rice paddy fields | Chinese cabbage | N2-fixation P-solubilization | 50% N | [118] |
| Rhodopseudomonas palustris spp. (2.45 × 108 CFU mL−1) | Liquid (Foliar) | Paddy soils | Rice | N/A | N/A | [119] |
N/A: not available; PGPS: plant growth-promoting substances.
3.2.2.1 In liquid form
One of the most common applications of PNSB is in liquid biofertilizers [120]. In this form, the bacteria are stored and supplied to plants as broth. The benefits of this procedure are that the inoculants are easy to make and store at a low cost in its additives, e.g., coconut juice, polyvinyl alcohol, xanthan gum, gelatin, mineral oil, horticultural oil, and Arabic gum, which can elongate the half-life of the cells within the products [121,122,123,124,125,126]. Horticultural oil is mainly added to liquid-based PNSB inoculants to maintain the cell for a long time (up to a month) at any temperature [123]. However, liquid inoculants may have some obstacles in transportation. In a study by Khuong et al. [55], a liquid application of PNSB, including R. palustris and R. harwoodiae, was used to supply rice in acid-sulfate soil and efficiently increased available P, thereby enhancing rice growth and grain yield.
3.2.2.2 In solid form
Unlike the liquid form, the solid form application of PNSB can simultaneously contain one or even more strains in a carrier material [94]. The PNSB’s survival was supported by an appropriate carrier material in this form. It gives the bacteria a safe environment to avoid adverse conditions and promotes a storage time of up to 6 months [127]. Carrier materials are usually made from formulations of dry, solid, or granular materials [127], such as peat, clay, compost, wheat bran, rice husk, wood ash, and sodium alginate, which are considered to have the highest efficiency [94,128,129,130]. Moreover, apart from the efficiency, the carrier materials should have low cost, enough availability in amounts, no toxicity to plants and inoculated bacteria, good moisture absorption, and good binding to the bacterial community [131]. In the meantime, wood ash can enhance the growth and activity of bacteria, i.e., higher expression of genes that contribute to metabolism and cell growth is induced [132]. In addition, palm oil may also be a fine carrier material since it can ameliorate soil fertility and plant development [133]. Furthermore, rice husk ash and spent coffee grounds, byproducts from rice production and the coffee industry, have features that promote plant growth. In detail, a rice husk ash carrier material has characteristics of good porosity and high water-holding capacity, which help maintain the bacteria and stimulate their growth inside the carrier material [134]. Concurrently, the spent coffee ground contains a high amount of K, Mg, and P [135], and it also assists the bacteria with good adhesion to soils [136]. In the study by Sakpirom et al. [131], a mixture of rubber wood ash, decanter cake rice husk ash, and spent coffee ground was used to carry PNSB strains of R. palustris and Rubrivivax gelatinosus, resulting in higher seedling growth than that of the liquid form of supplementation. Moreover, not only did a solid combination of six PNSB strains of R. palustris (TLS12, VNS19, VNS32, VNS62, and VNW95) and R. harwoodiae (TLW42) lead to a decrease of 20% in the chemical fertilizer used in this study via increasing available P content from P solubilization process, but also it reduced up to 36% of Mn content in rice grains [55].
3.2.3 Foliar application: A new and promising way to apply PNSB
A disadvantage of applying PNSB in either liquid or solid form is that the bacteria are easily affected by biological and nonbiological factors within the soil. Moreover, the addition of xenobiotic bacteria to the soil or the change in the density of indigenous bacteria in the soil may have tremendous effects on the microbial community and their structures. For these reasons, the advent of foliar fertilization has occurred. In the study by Yen et al. [119], rice was applied with PNSB by foliar fertilization. The result was robust, that is, root length, root dry biomass, productive tillers per plant, grains per plant, grain yield, 1,000-grain weight, and harvest index increased over the control treatment by 25, 57, 26, 38, 33, 1.6, and 41%, respectively. Unlike the nutrients providing function as the two above forms, this type of application is primarily aimed at preventing pathogens via biological control carried out by strains of PNSB. For instance, strains of PNSB could produce bioactive compounds such as EPS and lipopeptides to prevent diseases in crops. For EPS, R. palustris GJ-22 has been applied to tobacco plants to promote resistance against the tobacco mosaic virus [137]. This is due to the activity of an EPS called G-EPS, which can perform antiviral activity and stimulate the systematic resistance of tobacco plants [109]. Furthermore, the R. palustris GJ-22 strain has been used to prevent late blight in potatoes resulting from the oomycete Phytophthora infestans [138]. Not only does EPS secretion by PNSB benefit crops, but it can also boost their life under difficult conditions, as demonstrated by Nunkaew et al. [17], who found that R. palustris PP903 and TN114 produced EPS under high-salt conditions, extending their longevity. In the study by Nookongbut et al. [111], the PNSB strains, R. palustris KTSSR54, KTSWR26, KKSSG39, and KTSWR2, were applied on rice and showed activities against pathogens caused by fungi. Among them, the R. palustris KTSSR54 performed excellently on inhibitions against three types of diseases on rice, including brown spot (caused by Bipolaris oryzae NPT0508), leaf spot (caused by Curvularia lunata SPB0627), and rice blast (caused by Magnaporthe oryzae PTRC63), by releasing EPS [18]. For lipopeptides, Pseudomonas sp. can antagonize fungal pathogens on sugar beet and rice via lipopeptide production mechanisms [139,140]. Additionally, the GroEL protein, produced by the R. palustris PSB-06, can inhibit crop disease and boost plant resistance in rice against the M. oryzae [141]. Apart from solely protecting crops from pathogens, PNSB strains can work with other microorganisms to carry out antagonism against pathogens as well. For example, the combination of the yeast and the R. palustris can inhibit the grey mold disease due to the growth of Botrytis cinerea fungi [142]. Moreover, when the PNSB, R. faecalis, was paired with the Bacillus aerophilus, a high antifungal activity was found [143]. Ultimately, the above PNSB strains have great potential in being applied to the foliar application due to their high performance in either antagonizing pathogens or inducing plant resistance to them, particularly R. palustris KTSSR54 and GJ-22.
4 Future perspectives
Although PNSB have been widely studied in laboratories and greenhouses [120], their on-field performances have not been fully discovered [144]. In the field, the environment’s complexity and diversity caused by various biological or nonbiological factors may heavily affect the functions of the bacteria before they can deliver their benefits [127]. For all these reasons, biofertilizers made of PNSB have difficulties in commercialization. Therefore, ensuring PNSB’s survival and functions is a must. As a result, encapsulation is the next step in researching PNSB. In the study by Rohman et al. [130], mixing alginate with 4% (w/v) starch results in microcapsules whose entrapment efficiency is 70.83% (higher than 50.56% of the control beads) and spherical shape is maintained after drying. However, this encapsulation production has not been performed in the field. Additionally, appropriate frequencies and concentrations of PNSB biofertilizers applied to crops have not been standardized due to the diversity of data obtained from previous studies [145]. Thus, research on PNSB should concentrate on indigenous strains and factors that contribute to their survival in various conditions, such as saline, alluvial, and acidic soils.
5 Conclusions
This article revealed that there has been a great demand for the improvement of P in agriculture due to low P availability in the soils. However, applying P fertilizer is not an effective way to enhance the availability of P within the soil due to P being fixed by different compounds in soil types, and it may cause several severe impacts on the environment as well. Additionally, mechanisms of P mineralization and P solubilization processes have been reviewed. However, the P-solubilizing microbes encounter a problem in that they are vulnerable to the soil environment, while the PNSB appears to be a remarkably potent approach. This study was one of the first to summarize the current status of research on P-solubilizing PNSB and their applications and introduce a future outlook of applying P-solubilizing PNSB more effectively. The PNSB can form spores that make them more adaptable to the surrounding environment. In particular, they can survive and grow under various conditions (acidity, salinity, toxicity, etc.), common conditions in the Mekong River Delta, Vietnam, and they can also solubilize P without increasing soil acidity. Therefore, the P-solubilizing PNSB can reduce the dependence on chemical P fertilizers as biofertilizers to improve soil health and be in alignment with the goals of sustainable agriculture. However, although the PNSB can provide available P for crops from insoluble sources of soil P fractions, such as Fe-P, Al-P, and Ca-P, based on the improvement in soil P availability and decreases in insoluble P content, the accurate mechanisms of the P-solubilizing PNSB have not been thoroughly investigated up to now. Therefore, future studies should focus on the pathways and the metabolisms of P solubilization by PNSB. Moreover, the PNSB biofertilizers have been used in liquid and solid forms and newly sprayed to leaves instead of soil amendments. However, different studies used different concentrations of PNSB inoculants under different soil characteristics, regions, climates, crops, and so forth, leading to data from different research groups differing, though the results are all promising. Thus, PNSB should be investigated in relation to indigenous strains, how to keep them alive, and what their functions are.
-
Funding information: This research was funded by Vietnam National University HoChiMinh City (VNU-HCM) under grant number B2024-16-02.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results and approved the final version of the manuscript. NQK: conceptualization, writing – original draft; LTD: writing – original draft; LNTX: conceptualization, writing – original draft; LTQ: conceptualization, writing – review and editing; NKN: conceptualization, writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Vitousek PM, Porder S, Houlton BZ, Chadwick OA. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl. 2010 Jan;20(1):5–15. 10.1890/08-0127.1.Search in Google Scholar PubMed
[2] Huang J, Xu CC, Ridoutt BG, Wang XC, Ren PA. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J Clean Prod. 2017 Aug;159:171–9. 10.1016/j.jclepro.2017.05.008.Search in Google Scholar
[3] Ni Z, Wang S, Wang Y. Characteristics of bioavailable organic phosphorus in sediment and its contribution to lake eutrophication in China. Env Pollut. 2016 Dec;219:537–44. 10.1016/j.envpol.2016.05.087.Search in Google Scholar PubMed
[4] Schoumans OF, Bouraoui F, Kabbe C, Oenema O, van Dijk KC. Phosphorus management in Europe in a changing world. J Env Soc. 2015 Mar;44:180–92. 10.1007/s13280-014-0613-9.Search in Google Scholar PubMed PubMed Central
[5] Dhillon J, Torres G, Driver E, Figueiredo B, Raun WR. World phosphorus use efficiency in cereal crops. Agron J. 2017 Jul;109(4):1670–7. 10.2134/agronj2016.08.0483.Search in Google Scholar
[6] Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Fron Plant Sci. 2018;9:1473. 10.3389/fpls.2018.01473.Search in Google Scholar PubMed PubMed Central
[7] Farhadinejad T, Khakzad A, Jafari M, Shoaee Z, Khosrotehrani K, Nobari R, et al. The study of environmental effects of chemical fertilizers and domestic sewage on water quality of Taft region, Central Iran. Arab J Geosci. 24 Jan;7:221–9. 10.1007/s12517-012-0717-0.Search in Google Scholar
[8] Hartmann M, Frey B, Mayer J, Mäder P, Widmer F. Distinct soil microbial diversity under long-term organic and conventional farming. Multidiscip J Microb Ecol. 2015 May;9:1177–94. 10.1038/ismej.2014.210.Search in Google Scholar PubMed PubMed Central
[9] von Westarp S, Sandra Brown HS, Shah PB. Agricultural intensification and the impacts on soil fertility in the Middle Mountains of Nepal. Can J Soil Sci. 2004 Aug;84:323–32. 10.4141/S03-053.Search in Google Scholar
[10] Li H, Mollier A, Ziadi N, Shi Y, Parent LÉ, Morel C. The long-term effects of tillage practice and phosphorus fertilization on the distribution and morphology of corn root. Plant Soil. 2017 Mar;412(1):97–114. 10.1007/s11104-016-2925-y.Search in Google Scholar
[11] Metson GS, MacDonald GK, Haberman D, Nesme T, Bennett EM. Feeding the corn belt: opportunities for phosphorus recycling in US agriculture. Sci Total Env. 2016 Jan;542:1117–26. 10.1016/j.scitotenv.2015.08.047.Search in Google Scholar PubMed
[12] Xin X, Qin S, Zhang J, Zhu A, Yang W, Zhang X. Yield, phosphorus use efficiency and balance response to substituting long-term chemical fertilizer use with organic manure in a wheat-maize system. Field Crop Res. 2017 Jul;208:27–33. 10.1016/j.fcr.2017.03.011.Search in Google Scholar
[13] Khourchi S, Elhaissoufi W, Loum M, Ibnyasser A, Haddine M, Ghani R, et al. Phosphate solubilizing bacteria can significantly contribute to enhance P availability from polyphosphates and their use efficiency in wheat. Microbiol Res. 2022 Sep;262:127094. 10.1016/j.micres.2022.127094.Search in Google Scholar PubMed
[14] Emami S, Alikhani HA, Pourbabaee AA, Etesami H, Motasharezadeh B, Sarmadian F. Consortium of endophyte and rhizosphere phosphate solubilizing bacteria improves phosphorous use efficiency in wheat cultivars in phosphorus deficient soils. Rhizosphere. 2020 Jun;14:100196. 10.1016/j.rhisph.2020.100196.Search in Google Scholar
[15] Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010 Dec;60:579–98. 10.1007/s13213-010-0117-1.Search in Google Scholar
[16] Costa S, Ganzerli S, Rugiero I, Pellizzari S, Pedrini P, Tamburini E. Potential of Rhodobacter capsulatus grown in anaerobic-light or aerobic-dark conditions as bioremediation agent for biological wastewater treatments. Water. 2017 Feb;9(2):108. 10.3390/w9020108.Search in Google Scholar
[17] Nunkaew T, Kantachote D, Nitoda T, Kanzaki H, Ritchie RJ. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr Polym. 2015 Jan;115:334–41. 10.1016/j.carbpol.2014.08.099.Search in Google Scholar PubMed
[18] Nookongbut P, Kantachote D, Khuong NQ, Tantirungkij M. The biocontrol potential of acid-resistant Rhodopseudomonas palustris KTSSR54 and its exopolymeric substances against rice fungal pathogens to enhance rice growth and yield. Biol Control. 2020 Nov;150:104354. 10.1016/j.biocontrol.2020.104354.Search in Google Scholar
[19] Dhar K, Venkateswarlu K, Megharaj M. Anoxygenic phototrophic purple non-sulfur bacteria: tool for bioremediation of hazardous environmental pollutants. World J Microbiol Biotechnol. 2023 Oct;39(10):283. 10.1007/s11274-023-03729-7.Search in Google Scholar PubMed PubMed Central
[20] Hsu SH, Shen MW, Chen JC, Lur HS, Liu CT. The photosynthetic bacterium Rhodopseudomonas palustris strain PS3 exerts plant growth-promoting effects by stimulating nitrogen uptake and elevating auxin levels in expanding leaves. Front Plant Sci. 2021 Feb;12:573634. 10.3389/fpls.2021.573634.Search in Google Scholar PubMed PubMed Central
[21] Bunraksa T, Kantachote D, Chaiprapat S. The potential use of purple nonsulfur bacteria to simultaneously treat chicken slaughterhouse wastewater and obtain valuable plant growth promoting effluent and their biomass for agricultural application. Biocatal Agric Biotechnol. 2020 Sep;28:101721. 10.1016/j.bcab.2020.101721.Search in Google Scholar
[22] Sakarika M, Spanoghe J, Sui Y, Wambacq E, Grunert O, Haesaert G, et al. Purple non‐sulphur bacteria and plant production: benefits for fertilization, stress resistance and the environment. Microb Biotechnol. 2020 Sep;13(5):1336–65. 10.1111/1751-7915.13474.Search in Google Scholar PubMed PubMed Central
[23] Huu TN, Giau TT, Ngan PN, Van TT, Khuong NQ. Potential of phosphorus solubilizing purple nonsulfur bacteria isolated from acid sulfate soil in improving soil property, nutrient uptake, and yield of pineapple (Ananas comosus L. Merrill) under acidic stress. Appl Environ Soil Sci. 2022 Nov;2022:8693479. 10.1155/2022/8693479.Search in Google Scholar
[24] Xuan LN, Huyen NP, Thu LT, Thuy VT, Tuan LM, Quang LT, et al. Supplementation of P-solubilizing purple nonsulfur bacteria, Rhodopseudomonas palustris improved soil fertility, P nutrient, growth, and yield of Cucumis melo L. Open Agric. 2024 Jan;9(1):20220247. 10.1515/opag-2022-0247.Search in Google Scholar
[25] Wang Y, Peng S, Hua Q. The long-term effects of using phosphate-solubilizing bacteria and photosynthetic bacteria as biofertilizers on peanut yield and soil bacteria community. Front Microbiol. 2021 Jul;12:693535. 10.3389/fmicb.2021.693535.Search in Google Scholar PubMed PubMed Central
[26] Iwai R, Uchida S, Yamaguchi S, Sonoda F, Tsunoda K, Nagata H, et al. Effects of seed bio-priming by purple non-sulfur bacteria (PNSB) on the root development of rice. Microorganisms. 2022 Nov;10(11):2197. 10.3390/microorganisms10112197.Search in Google Scholar PubMed PubMed Central
[27] Johnston AE, Poulton PR. Phosphorus in agriculture: A review of results from 175 years of research at Rothamsted, UK. J Env Qual. 2019 Sep;48(5):1133–44. 10.2134/jeq2019.02.0078.Search in Google Scholar PubMed
[28] Thakur D, Kaushal R, Shyam V. Phosphate solubilising microorganisms: role in phosphorus nutrition of crop plants – a review. Agric Rev. 2014;35(3):159–71. 10.5958/0976-0741.2014.00903.9.Search in Google Scholar
[29] Percival LME, Bond DPG, Rakociński M, Marynowski L, Hood AVS, Adatte T, et al. Phosphorus-cycle disturbances during the Late Devonian anoxic events. Glob Planet Change. 2020 Jan;184:103070. 10.1016/j.gloplacha.2019.103070.Search in Google Scholar
[30] Khan MD, Zaidi A, Ahmad E. Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms. In: Khan M, Zaidi A, Musarrat J, editors. Phosphate solubilizing microorganisms. Switzerland: Springer; 2014. p. 31–62. 10.1007/978-3-319-08216-5_2.Search in Google Scholar
[31] Tian J, Ge F, Zhang D, Deng S, Liu X. Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology. 2021 Feb;10(2):158. 10.3390/biology10020158.Search in Google Scholar PubMed PubMed Central
[32] Cong WF, Suriyagoda LDB, Lambers H. Tightening the phosphorus cycle through phosphorus-efficient crop genotypes. Trends Plant Sci. 2020 Oct;25(10):967–75. 10.1016/j.tplants.2020.04.013.Search in Google Scholar PubMed
[33] Ghosal PK, Chakraborty T. Comparative solubility study of four phosphatic fertilizers in different solvents and the effect of soil. Resour Env. 2012 Aug;2(4):175–9. 10.5923/j.re.20120204.07.Search in Google Scholar
[34] Ahmed W, Jing H, Kaillou L, Qaswar M, Khan MN, Jin C, et al. Changes in phosphorus fractions associated with soil chemical properties under long-term organic and inorganic fertilization in paddy soils of southern China. PLoS One. 2019 May;14(5):e0216881. 10.1371/journal.pone.0216881.Search in Google Scholar PubMed PubMed Central
[35] Yan X, Wang D, Zhang G, Bo L, Peng X. Soil phosphorous accumulation in long-term P fertilization paddy field and its environmental effects. Chin J Eco-Agric. 2013;21(4):393–400. 10.3724/SP.J.1011.2013.00393.Search in Google Scholar
[36] Mohanty S, Paikaray NK, Rajan AR. Availability and uptake of phosphorus from organic manures in groundnut (Arachis hypogea L.)–corn (Zea mays L.) sequence using radio tracer technique. Geoderma. 2006 Aug;133(3–4):225–30. 10.1016/j.geoderma.2005.07.009.Search in Google Scholar
[37] Reddy DD, Rao SA, Singh M. Changes in P fractions and sorption in an Alfisol following crop residues application. J Plant Nutr Soil Sci. 2005 Apr;168(2):241–7. 10.1002/jpln.200421444.Search in Google Scholar
[38] Xu Z. Effect of long-term located fertilization on the yields of rice and wheat and soil nutrient. Acta Agric Zhejiangensis. 2009;21(5):485–9.Search in Google Scholar
[39] Zhao Y, Wang P, Li J, Chen Y, Ying X, Liu S. The effects of two organic manures on soil properties and crop yields on a temperate calcareous soil under a wheat–maize cropping system. Eur J Agron. 2009 Jul;31(1):36–42. 10.1016/j.eja.2009.03.001.Search in Google Scholar
[40] Xu X, Mao X, Van Zwieten L, Niazi NK, Lu K, Bolan NS, et al. Wetting-drying cycles during a rice-wheat crop rotation rapidly (im) mobilize recalcitrant soil phosphorus. J Soils Sediment. 2020 Nov;20(11):3921–30. 10.1007/s11368-020-02712-1.Search in Google Scholar
[41] Cross AF, Schlesinger WH. A literature review and evaluation of the Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma. 1995 Jan;64(3–4):197–214. 10.1016/0016-7061(94)00023-4.Search in Google Scholar
[42] Prietzel J, Harrington G, Häusler W, Heister K, Werner F, Klysubun W. Reference spectra of important adsorbed organic and inorganic phosphate binding forms for soil P speciation using synchrotron-based K-edge XANES spectroscopy. J Synchrotron Radiat. 2016 Mar;23(2):532–44. 10.1107/S1600577515023085.Search in Google Scholar PubMed
[43] Menezes-Blackburn D, Giles C, Darch T, George TS, Blackwell M, Stutter M, et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: a review. Plant Soil. 2018 Jun;427(1):5–16. 10.1007/s11104-017-3362-2.Search in Google Scholar PubMed PubMed Central
[44] Pierzynski GM, McDowell RW, Sims JT. Chemistry, cycling, and potential movement of inorganic phosphorus in soils. In: Sims JT, Sharpley AN, editors. Phosphorus: agriculture and the environment. America: American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc; 2005. p. 53–86. 10.2134/agronmonogr46.c3.Search in Google Scholar
[45] Syers J, Johnston A, Curtin D. Efficiency of soil and fertilizer phosphorus use. FAO Fertil Plant Nutr Bull. 2008;18(108):5–50.Search in Google Scholar
[46] Menezes-Blackburn D, Zhang H, Stutter M, Giles C, Darch T, George TS, et al. A holistic approach to understanding the desorption of phosphorus in soils. Env Sci Technol. 2016 Apr;50(7):3371–81. 10.1021/acs.est.5b05395.Search in Google Scholar PubMed
[47] Boitt G, Simpson ZP, Tian J, Black A, Wakelin SA, Condron LM. Plant biomass management impacts on short-term soil phosphorus dynamics in a temperate grassland. Biol Fertil Soils. 2018 Apr;54:397–409. 10.1007/s00374-018-1269-6.Search in Google Scholar
[48] Yadav BK, Verma A. Phosphate solubilization and mobilization in soil through microorganisms under arid ecosystems. In: Ali M, editor. The functioning of ecosystems. Rijeka: Intech; 2012. p. 93–108. 10.5772/35917.Search in Google Scholar
[49] Turner BL, Paphazy MJ, Haygarth PM, McKelvie ID. Inositol phosphates in the environment. Philos Trans R Soc B: Biol Sci. 2002 Apr;357(1420):449–69. 10.1098/rstb.2001.0837.Search in Google Scholar PubMed PubMed Central
[50] Rawat P, Das S, Shankhdhar D, Shankhdhar SC. Phosphate-solubilizing microorganisms: mechanism and their role in phosphate solubilization and uptake. J Soil Sci Plant Nutr. 2021 Mar;21(1):49–68. 10.1007/s42729-020-00342-7.Search in Google Scholar
[51] Aseri GK, Jain N, Tarafdar JC. Hydrolysis of organic phosphate forms by phosphatases and phytase producing fungi of arid and semi-arid soils of India. Am Eurasian J Agric Env Sci. 2009 Dec;5:564–70.Search in Google Scholar
[52] Maougal RT, Brauman A, Plassard C, Abadie J, Djekoun A, Drevon JJ. Bacterial capacities to mineralize phytate increase in the rhizosphere of nodulated common bean (Phaseolus vulgaris) under P deficiency. Eur J Soil Biol. 2014 May;62:8–14. 10.1016/j.ejsobi.2014.02.006.Search in Google Scholar
[53] Arif MS, Shahzad SM, Yasmeen T, Riaz M, Ashraf M, Ashraf MA, et al. Improving plant phosphorus (P) acquisition by phosphate-solubilizing bacteria. In: Naeem M, Ansari A, Gill S, editors. Essential plant nutrients. Switzerland: Springer; 2017. p. 513–56. 10.1007/978-3-319-58841-4_21.Search in Google Scholar
[54] George TS, Quiquampoix H, Simpson RJ, Richardson AE. Interactions between phytases and soil constituents: implications for the hydrolysis of inositol phosphates. In: Turner B, Richardson A, Mullaney E, editors. Inositol phosphates, linking agriculture and the environment. New Orleans: CABI; 2007. p. 221–41. 10.1079/9781845931520.0221.Search in Google Scholar
[55] Khuong NQ, Kantachote D, Huu TN, Nhan TC, Nguyen PC, Van TB, et al. Use of potent acid resistant strains of Rhodopseudomonas spp. in Mn-contaminated acidic paddies to produce safer rice and improve soil fertility. Soil Tillage Res. 2022 Jul;221:105393. 10.1016/j.still.2022.105393.Search in Google Scholar
[56] Clarholm M, Skyllberg U, Rosling A. Organic acid induced release of nutrients from metal-stabilized soil organic matter-the unbutton model. Soil Biol Biochem. 2015 May;84:168–76. 10.1016/j.soilbio.2015.02.019.Search in Google Scholar
[57] Richardson AE, George TS, Hens M, Simpson RJ. Utilisation of soilorganic phosphorus by higher plants. In: Turner BL, Frossard E, Baldwin DS, editors. Organic phosphorus in the environment. Wallingford, UK: CABI; 2005. p. 165–84. 10.1079/9780851998220.0165.Search in Google Scholar
[58] Wan W, Hao X, Xing Y, Liu S, Zhang X, Li X, et al. Spatial differences in soil microbial diversity caused by pH‐driven organic phosphorus mineralization. Land Degrad Dev. 2021 Jan;32(2):766–76. 10.1002/ldr.3734.Search in Google Scholar
[59] Jiang Y, Zhao X, Zhou Y, Ding C. Effect of the phosphate solubilization and mineralization synergistic mechanism of Ochrobactrum sp. on the remediation of lead. Env Sci Pollut Res. 2022 Aug;29(38):58037–52. 10.1007/s11356-022-19960-y.Search in Google Scholar PubMed
[60] Di Simine CD, Sayer JA, Gadd GM. Solubilization of zinc phosphate by a strain of Pseudomonas fluorescens isolated from a forest soil. Biol Fertil Soils. 1998 Nov;28:87–94.10.1007/s003740050467Search in Google Scholar
[61] Gupta N, Sabat J, Parida R, Kerkatta D. Solubilization of tricalcium phosphate and rock phosphate by microbes isolated from chromite, iron and manganese mines. Acta Bot Croat. 2007 Oct;66(2):197–204.Search in Google Scholar
[62] Khan MS, Ahmad E, Zaidi A, Oves M. Functional aspect of phosphate-solubilizing bacteria: importance in crop production. In: Maheshwari D, Saraf M, Aeron A, editors. Bacteria in agrobiology: crop productivity. Heidelberg, Berlin: Springer; 2013. p. 237–65. 10.1007/978-3-642-37241-4_10.Search in Google Scholar
[63] Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus. 2013 Dec;2:587. 10.1186/2193-1801-2-587.Search in Google Scholar PubMed PubMed Central
[64] Song OR, Lee SJ, Lee YS, Lee SC, Kim KK, Choi YL. Solubilization of insoluble inorganic phosphate by Burkholderia cepacia DA23 isolated from cultivated soil. Braz J Microbiol. 2008;39(1):151–56. 10.1590/S1517-838220080001000030.Search in Google Scholar PubMed PubMed Central
[65] Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA. Plant growth promotion by phosphate solubilizing fungi-current perspective. Arch Agron Soil Sci. 2010 Feb;56:73–98. 10.1080/03650340902806469.Search in Google Scholar
[66] Maliha R, Samina K, Najma A, Sadia A, Farooq L. Organic acid production and phosphate solubilization by phosphate solubilizing microorganisms under in vitro conditions. Pak J Biol Sci. 2004;7(2):187–96. 10.3923/pjbs.2004.187.196.Search in Google Scholar
[67] Marra LM, Fonsêca Sousa Soares CR, de Oliveira SM, Ferreira PAAA, Soares BL, Carvalho RF, et al. Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant Soil. 2012 Mar;357:289–307. 10.1007/s11104-012-1157-z.Search in Google Scholar
[68] Kim KY, McDonald GA, Jordan D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol Fertil Soils. 1997 May;24:347–52. 10.1007/s003740050256.Search in Google Scholar
[69] Wei Y, Zhao Y, Shi M, Cao Z, Lu Q, Yang T, et al. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour Technol. 2018 Jan;247:190–9. 10.1016/j.biortech.2017.09.092.Search in Google Scholar PubMed
[70] Yang T, Li L, Wang B, Tian J, Shi F, Zhang S, et al. Isolation, mutagenesis, and organic acid secretion of a highly efficient Phosphate-solubilizing fungus. Front Microbiol. 2022 Apr;13:793122. 10.3389/fmicb.2022.793122.Search in Google Scholar PubMed PubMed Central
[71] Pradhan N, Shukla LB. Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr J Biotechnol. 2006;5(10):850–4.Search in Google Scholar
[72] Trivedi P, Sa T. Pseudomonas corrugata (NRRL B-30409) mutants increased phosphate solubilization, organic acid production, and plant growth at lower temperatures. Curr Microbiol. 2008 Feb;56:140–4. 10.1007/s00284-007-9058-8.Search in Google Scholar PubMed
[73] Rafi MM, Krishnaveni MS, Charyulu PB. Phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture. In: Buddolla V, editor. Recent developments in applied microbiology and biochemistry. Cambridge, Massachusetts: Academic Press; 2019. p. 223–33. 10.1016/B978-0-12-816328-3.00017-9.Search in Google Scholar
[74] Hii YS, San CY, Lau SW, Danquah MK. Isolation and characterisation of phosphate solubilizing microorganisms from peat. Biocatal Agric Biotechnol. 2020 Jul;26:101643. 10.1016/j.bcab.2020.101643.Search in Google Scholar
[75] Anu A, Kundu BS. Development and characterization of lacZ marked strains of phosphate solubilizing bacteria. Indian J Microbiol. 2005;45:261–3.Search in Google Scholar
[76] Kouam ID, Mabah J, Germain Ntsoli P, Tchamani L, Yaouba A, Katte B, et al. Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon. Open Agric. 2023 Mar;8(1):20220154. 10.1515/opag-2022-0154.Search in Google Scholar
[77] Wan W, Qin Y, Wu H, Zuo W, He H, Tan J, et al. Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front Microbiol. 2020 Apr;11:510229. 10.3389/fmicb.2020.00752.Search in Google Scholar PubMed PubMed Central
[78] Amri M, Rjeibi MR, Gatrouni M, Mateus DM, Asses N, Pinho HJ, et al. Isolation, identification, and characterization of phosphate-solubilizing bacteria from Tunisian soils. Microorganisms. 2023 Mar;11(3):783. 10.3390/microorganisms11030783.Search in Google Scholar PubMed PubMed Central
[79] Aliyat FZ, Maldani M, El Guilli M, Nassiri L, Ibijbijen J. Isolation and characterization of phosphate solubilizing bacteria from phosphate solid sludge of the moroccan phosphate mines. Open Agric J. 2020 Mar;14(1):16–24. 10.2174/1874331502014010016.Search in Google Scholar
[80] Dhankhar R, Sheoran S, Dhaka A, Soni R. The role of phosphorus solubilizing bacteria (PSB) in soil management-an overview. Int J Dev Res. 2013;3(9):31–6.Search in Google Scholar
[81] Timofeeva A, Galyamova M, Sedykh S. Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plants. 2022 Aug;11(16):2119. 10.3390/plants11162119.Search in Google Scholar PubMed PubMed Central
[82] Ramakrishna W, Yadav R, Li K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl Soil Ecol. 2019 Jun;138:10–8. 0.1016/j.apsoil.2019.02.019.Search in Google Scholar
[83] Zhang T, Hu F, Ma L. Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth. Open Life Sci. 2019 Jul;14:246–54. 10.1515/biol-2019-0028.Search in Google Scholar PubMed PubMed Central
[84] Gross A, Lin Y, Weber PK, Pett‐Ridge J, Silver WL. The role of soil redox conditions in microbial phosphorus cycling in humid tropical forests. Ecology. 2020 Feb;101(2):e02928. 10.1002/ecy.2928.Search in Google Scholar PubMed
[85] Liang JL, Liu J, Jia P, Yang TT, Zeng QW, Zhang SC, et al. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. Multidiscip J Microb Ecol. 2020 Jun;14(6):1600–13. 10.1038/s41396-020-0632-4.Search in Google Scholar PubMed PubMed Central
[86] Baliah NT, Pandiarajan G, Kumar BM. Isolation, identification and characterization of phosphate solubilizing bacteria from different crop soils of Srivilliputtur Taluk, Virudhunagar District, Tamil Nadu. Trop Ecol. 2016 Sep;57(3):465–74.Search in Google Scholar
[87] Behera BC, Yadav H, Singh SK, Mishra RR, Sethi BK, Dutta SK, et al. Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J Genet Eng Biotechnol. 2017 Jun;15:169–78. 10.1016/j.jgeb.2017.01.003.Search in Google Scholar PubMed PubMed Central
[88] Sanchez-Gonzalez ME, Mora-Herrera ME, Wong-Villarreal A, De La Portilla-López N, Sanchez-Paz L, Lugo J, et al. Effect of pH and carbon source on phosphate solubilization by bacterial strains in Pikovskaya medium. Microorganisms. 2022 Dec;11(1):49. 10.3390/microorganisms11010049.Search in Google Scholar PubMed PubMed Central
[89] Khuong NQ, Kantachote D, Dung NTT, Huu TN, Thuc LV, Thu LTM, et al. Potential of potent purple nonsulfur bacteria isolated from rice-shrimp systems to ameliorate rice (Oryza sativa L.) growth and yield in saline acid sulfate soil. J Plant Nutr. 2023 Feb;46(3):473–94. 10.1080/01904167.2022.2087089.Search in Google Scholar
[90] Maeda I. Potential of phototrophic purple nonsulfur bacteria to fix nitrogen in rice fields. Microorganisms. 2021 Dec;10(1):28. 10.3390/microorganisms10010028.Search in Google Scholar PubMed PubMed Central
[91] Morton LW, Nguyen NK, Demyan MS. Salinity and acid sulfate soils of the Vietnam Mekong Delta: Agricultural management and adaptation. J Soil Water Conserv. 2023 Jul;78(4):85A–92A. 10.2489/jswc.2023.0321A.Search in Google Scholar
[92] Harada N, Nishiyama M, Otsuka S, Matsumoto S. Effects of inoculation of phototrophic purple bacteria on grain yield of rice and nitrogenase activity of paddy soil in a pot experiment. Soil Sci Plant Nutr. 2005 Jun;51:361–7. 10.1111/j.1747-0765.2005.tb00041.x.Search in Google Scholar
[93] Khuong NQ, Huu TN, Thuc LV, Thu LTM, Xuan DT, Quang LT, et al. Two strains of Luteovulum sphaeroides (purple nonsulfur bacteria) promote rice cultivation in saline soils by increasing available phosphorus. Rhizosphere. 2021 Dec;20:100456. 10.1016/j.rhisph.2021.100456.Search in Google Scholar
[94] Kantha T, Kantachote D, Klongdee N. Potential of biofertilizers from selected Rhodopseudomonas palustris strains to assist rice (Oryza sativa L. subsp. indica) growth under salt stress and to reduce greenhouse gas emissions. Ann Microbiol. 2015 Dec;65:2109–18. 10.1007/s13213-015-1049-6.Search in Google Scholar
[95] Khuong NQ, Kantachote D, Nookongbut P, Onthong J, Xuan LN, Sukhoom A. Mechanisms of acid-resistant Rhodopseudomonas palustris strains to ameliorate acidic stress and promote plant growth. Biocatal Agric Biotechnol. 2020 Mar;24:101520. 10.1016/j.bcab.2020.101520.Search in Google Scholar
[96] Lo KJ, Lin SS, Lu CW, Kuo CH, Liu CT. Whole-genome sequencing and comparative analysis of two plant-associated strains of Rhodopseudomonas palustris (PS3 and YSC3). Sci Rep. 2018 Aug;8(1):12769. 10.1038/s41598-018-31128-8.Search in Google Scholar PubMed PubMed Central
[97] Tian D, Niu S. A global analysis of soil acidification caused by nitrogen addition. Env Res Lett. 2015 Feb;10(2):024019. 10.1088/1748-9326/10/2/024019.Search in Google Scholar
[98] Okur B, Örçen N. Soil salinization and climate change. In: Prasad MNV, Pietrzykowski M, editors. Climate change and soil interactions. Amsterdam, The Netherlands: Elsevier; 2020. p. 331–50. 10.1016/B978-0-12-818032-7.00012-6.Search in Google Scholar
[99] Holguin G, Vazquez P, Bashan Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol Fertil Soils. 2001 Apr;33:265–78. 10.1007/s003740000319.Search in Google Scholar
[100] Crovadore J, Xu S, Chablais R, Cochard B, Lukito D, Calmin G, et al. Metagenome-assembled genome sequence of Rhodopseudomonas palustris strain ELI 1980, commercialized as a biostimulant. Genome Announc. 2017;5:e00221–17. 10.1128/genomeA.00221-17.Search in Google Scholar PubMed PubMed Central
[101] Hiraishi A, Kitamura H. Distribution of phototrophic purple nonsulfur bacteria in activated sludge systems and other aquatic environments. Nippon Suisan Gakkaishi. 1985;50(11):1929–37. 10.2331/suisan.50.1929.Search in Google Scholar
[102] Oda Y, Wanders W, Huisman LA, Meijer WG, Gottschal JC, Forney LJ. Genotypic and phenotypic diversity within species of purple nonsulfur bacteria isolated from aquatic sediments. Appl Env Microbiol. 2002 Jul;68(7):3467–77. 10.1128/AEM.68.7.3467-3477.2002.Search in Google Scholar PubMed PubMed Central
[103] Lee KH, Koh RH, Song HG. Enhancement of growth and yield of tomato by Rhodopseudomonas sp. under greenhouse conditions. J Microbiol. 2008 Dec;46:641–6. 10.1007/s12275-008-0159-2.Search in Google Scholar PubMed
[104] Wu Y, Liao W, Yu J. 5-Aminolevulinic acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front Plant Sci. 2018 May;9:340044. 10.3389/fpls.2018.00635.Search in Google Scholar PubMed PubMed Central
[105] Li L, Song W, Deng C, Zhang D, Al-Misned FA, Mortuza MG, et al. Effects of pH and salinity on adsorption of hypersaline photosynthetic microbial mat exopolymers to goethite: A study using a quartz crystal microbalance and fluorescence spectroscopy. Geomicrobiol J. 2016 Mar;33(3–4):332–7. 10.1080/01490451.2015.1052120.Search in Google Scholar
[106] Rajkumar M, Ae N, Prasad MN, Freitas H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010 Mar;28(3):142–9. 10.1016/j.tibtech.2009.12.002.Search in Google Scholar PubMed
[107] Aberathna AA, Satharasinghe DA, Jayasooriya AP, Jinadasa RN, Manopriya S, Jayaweera BP, et al. Increasing the bioavailability of phosphate by using microorganisms. Int J Agron. 2022;2022(1):4305501. 10.1155/2022/4305501.Search in Google Scholar
[108] Wu J, Wang Y, Lin X. Purple phototrophic bacterium enhances stevioside yield by Stevia rebaudiana bertoni via foliar spray and rhizosphere irrigation. PLoS One. 2013 Jun;8(6):e67644. 10.1371/journal.pone.0067644.Search in Google Scholar PubMed PubMed Central
[109] Zhai Z, Chen A, Zhou H, Zhang D, Du X, Liu Q, et al. Structural characterization and functional activity of an exopolysaccharide secreted by Rhodopseudomonas palustris GJ-22. Int J Biol Macromol. 2021 Jan;167:160–8. 10.1016/j.ijbiomac.2020.11.165.Search in Google Scholar PubMed
[110] Khuong NQ, Kantachote D, Onthong J, Xuan LNT, Sukhoom A. Enhancement of rice growth and yield in actual acid sulfate soils by potent acid-resistant Rhodopseudomonas palustris strains for producing safe rice. Plant Soil. 2018 Aug;429(1):483–501. 10.1007/s11104-018-3705-7.Search in Google Scholar
[111] Nookongbut P, Kantachote D, Khuong NQ, Sukhoom A, Tantirungkij M, Limtong S. Selection of acid-resistant purple nonsulfur bacteria from peat swamp forests to apply as biofertilizers and biocontrol agents. J Soil Sci Plant Nutr. 2019 Sep;19(3):488–500. 10.1007/s42729-019-00044-9.Search in Google Scholar
[112] Rana G, Meikap S, Mondol M, Bose PP. Green-fertilizer, Rhodospirillum rubrum, for agricultural development on fly-ash without any toxic metal ion release. Basic Res J Agric Sci Rev. 2016;5(8):109–17.Search in Google Scholar
[113] O’Callaghan M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl Microbiol Biotechnol. 2016 Jul;100:5729–46. 10.1007/s00253-016-7590-9.Search in Google Scholar PubMed PubMed Central
[114] Potts M. Desiccation tolerance of prokaryotes. Microbiol Mol Biol Rev. 1994 Dec;58:755–805. 10.1128/mr.58.4.755-805.1994.Search in Google Scholar PubMed PubMed Central
[115] Berninger T, González López Ó, Bejarano A, Preininger C, Sessitsch A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb Biotechnol. 2018 Mar;11:277–301. 10.1111/1751-7915.12880.Search in Google Scholar PubMed PubMed Central
[116] Khuong NQ, Thuc LV, Quang LT, Huu TN, Xuan DT, Duc HH, et al. Effects of biofertilizer supplementation, Rhodopseudomonas spp., on nitrogen and phosphorus uptakes, growth, and yield of sesame (Sesamum indicum L.) on salt-affected soil. J Plant Nutr. 2024 Jan;47(1):1–7. 10.1080/01904167.2023.2278646.Search in Google Scholar
[117] Khuong NQ, Trong ND, Quang LT, Xuan LN, Phong NT. The potency of a liquid biofertilizer containing bacterial strains of Rhodopseudomonas spp. on recovery of soil properties damaged by Al3+ and Fe2+ toxins and enhancement of rice yield in acid sulfate soil. Int J Phytoremediat. 2024 Mar;26(4):535–45. 10.1080/15226514.2023.2253913.Search in Google Scholar PubMed
[118] Wong WT, Tseng CH, Hsu SH, Lur HS, Mo CW, Huang CN, et al. Promoting effects of a single Rhodopseudomonas palustris inoculant on plant growth by Brassica rapa chinensis under low fertilizer input. Microb Env. 2014;29(3):303–13. 10.1264/jsme2.ME14056.Search in Google Scholar
[119] Yen KS, Sundar LS, Chao YY. Foliar application of Rhodopseudomonas palustris enhances the rice crop growth and yield under field conditions. Plants. 2022 Sep;11(19):2452. 10.3390/plants11192452.Search in Google Scholar PubMed PubMed Central
[120] Lee SK, Lur HS, Liu CT. From lab to farm: Elucidating the beneficial roles of photosynthetic bacteria in sustainable agriculture. Microorganisms. 2021 Nov;9(12):2453. 10.3390/microorganisms9122453.Search in Google Scholar PubMed PubMed Central
[121] Anith K, Vaishakhi A, Viswanathan A, Varkey S, Aswini S. Population dynamics and efficiency of coconut water based liquid formulation of Pseudomonas fluorescens AMB-8. J Trop Agric. 2016;54(2):184–9.Search in Google Scholar
[122] Bernabeu PR, García SS, López AC, Vio SA, Carrasco N, Boiardi JL, et al. Assessment of bacterial inoculant formulated with Paraburkholderia tropica to enhance wheat productivity. World J Microbiol Biotechnol. 2018 Jun;34:81. 10.1007/s11274-018-2461-4.Search in Google Scholar PubMed
[123] Lee SK, Lur HS, Lo KJ, Cheng KC, Chuang CC, Tang SJ, et al. Evaluation of the effects of different liquid inoculant formulations on the survival and plant-growth-promoting efficiency of Rhodopseudomonas palustris strain PS3. Appl Microbiol Biotechnol. 2016 Sep;100:7977–87. 10.1007/s00253-016-7582-9.Search in Google Scholar PubMed
[124] Lobo CB, Juárez Tomás MS, Viruel E, Ferrero MA, Lucca ME. Development of low-cost formulations of plant growth promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol Res. 2019 Feb;219:12–25. 10.1016/j.micres.2018.10.012.Search in Google Scholar PubMed
[125] Pastor-Bueis R, Mulas R, Gómez X, González-Andrés F. Innovative liquid formulation of digestates for producing a biofertilizer based on Bacillus siamensis: Field testing on sweet pepper. J Plant Nutr Soil Sci. 2017 Dec;180:748–58. 10.1002/jpln.201700200.Search in Google Scholar
[126] Valetti L, Angelini J, Taurian T, Ibañez F, Muñoz V, Anzuay S, et al. Development and field evaluation of liquid inoculants with native Bradyrhizobial strains for peanut production. Afr Crop Sci J. 2016 Feb;24(1):1–13. 10.4314/acsj.v24i1.1.Search in Google Scholar
[127] Herrmann L, Lesueur D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl Microbiol Biotechnol. 2013 Oct;97(20):8859–73. 10.1007/s00253-013-5228-8.Search in Google Scholar PubMed
[128] Abd El-Fattah DA, Eweda WE, Zayed MS, Hassanein MK. Effect of carrier materials, sterilization method, and storage temperature on survival and biological activities of Azotobacter chroococcum inoculant. Ann Agric Sci. 2013 Dec;58(2):111–8. 10.1016/j.aoas.2013.07.001.Search in Google Scholar
[129] Arora NK, Tiwari S, Singh R. Comparative study of different carriers inoculated with nodule forming and free living plant growth promoting bacteria suitable for sustainable agriculture. J Plant Pathol Microbiol. 2014 Jan;5(2):1000229. 10.4172/2157-7471.1000229.Search in Google Scholar
[130] Rohman S, Kaewtatip K, Kantachote D, Tantirungkij M. Encapsulation of Rhodopseudomonas palustris KTSSR54 using beads from alginate/starch blends. J Appl Polym Sci. 2021 Mar;138(12):50084. 10.1002/app.50084.Search in Google Scholar
[131] Sakpirom J, Nunkaew T, Khan E, Kantachote D. Optimization of carriers and packaging for effective biofertilizers to enhance Oryza sativa L. growth in paddy soil. Rhizosphere. 2021 Sep;19:100383. 10.1016/j.rhisph.2021.100383.Search in Google Scholar
[132] Bang-Andreasen T, Anwar MZ, Lanzén A, Kjøller R, Rønn R, Ekelund F, et al. Total RNA sequencing reveals multilevel microbial community changes and functional responses to wood ash application in agricultural and forest soil. FEMS Microbiol Ecol. 2020 Mar;96:1–13. 10.1093/femsec/fiaa016.Search in Google Scholar PubMed PubMed Central
[133] Embrandiri A, Rupani PF, Shahadat M, Singh RP, Ismail SA, Ibrahim MH, et al. The phytoextraction potential of selected vegetable plants from soil amended with oil palm decanter cake. Int J Recycl Org Waste Agric. 2017 Mar;6(1):37–45. 10.1007/s40093-016-0150-6.Search in Google Scholar
[134] Ogbo FC, Odo MO. Potential of rice husk and cassava peel as carriers for bio-fertilizer production. Niger J Biotechnol. 2011;23:1–4.Search in Google Scholar
[135] Cruz R, Cardoso MM, Fernandes L, Oliveira M, Mendes E, Baptista P, et al. Espresso coffee residues: A valuable source of unextracted compounds. J Agric Food Chem. 2012 Aug;60:7777–84. 10.1021/jf3018854.Search in Google Scholar PubMed
[136] Cervera-Mata A, Pastoriza S, Rufian-Henares JA, Parraga J, Martín-García JM, Delgado G. Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch Agron Soil Sci. 2018 May;64(6):790–804. 10.1080/03650340.2017.1387651.Search in Google Scholar
[137] Su P, Zhang D, Zhang Z, Chen A, Hamid MR, Li C, et al. Characterization of Rhodopseudomonas palustris population dynamics on tobacco phyllosphere and induction of plant resistance to Tobacco mosaic virus. Microb Biotechnol. 2019 Nov;12(6):1453–63. 10.1111/1751-7915.13486.Search in Google Scholar PubMed PubMed Central
[138] Zhang X, Li X, Zhang Y, Chen Y, Tan X, Su P, et al. Integrated control of potato late blight with a combination of the photosynthetic bacterium Rhodopseudomonas palustris strain GJ-22 and fungicides. J Int Organ Biol Control. 2020 Oct;65(5):635–45. 10.1007/s10526-020-10026-x.Search in Google Scholar
[139] Nielsen TH, Thrane C, Christophersen C, Anthoni U, Sørensen J. Structure, production characteristics and fungal antagonism of tensin-a new antifungal cyclic lipopeptide from Pseudomonas fluorescens strain 96.578. J Appl Microbiol. 2000 Dec;89(6):992–1001. 10.1046/j.1365-2672.2000.01201.x.Search in Google Scholar PubMed
[140] Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev. 2010 Nov;34(6):1037–62. 10.1111/j.1574-6976.2010.00221.x.Search in Google Scholar PubMed
[141] Wu X, Chen Y, Li C, Zhang X, Tan X, Lv L, et al. GroEL protein from the potential biocontrol agent Rhodopseudomonas palustris enhances resistance to rice blast disease. Pest Manag Sci. 2021 Dec;77(12):5445–53. 10.1002/ps.6584.Search in Google Scholar PubMed
[142] Ristić D, Vučurović I, Aleksić G, Nikolić B, Đurović S, Starović M. Application of different combinations of lactic acid, phototrophic bacteria and yeast mixtures in control of seed and seedlings pathogens of tomato and pepper. Pestic Phytomed. 2021;36(2):73–82. 10.2298/PIF2102073R.Search in Google Scholar
[143] Wei H, Okunishi S, Yoshikawa T, Kamei Y, Dawwoda MA, Santander-De Leon SMS, et al. Synergistic effect of photosynthetic bacteria and isolated bacteria in their antifungal activities against root rot fungi. Biocontrol Sci. 2016;21(3):173–7. 10.4265/bio.21.173.Search in Google Scholar PubMed
[144] Martınez-Hidalgo P, Maymon M, Pule-Meulenberg F, Hirsch AM. Engineering root microbiomes for healthier crops and soils using beneficial, environmentally safe bacteria. Can J Microbiol. 2019;65(2):91–104. 10.1139/cjm-2018-0315.Search in Google Scholar PubMed
[145] Sundar LS, Chao YY. Potential of purple non-sulfur bacteria in sustainably enhancing the agronomic and physiological performances of rice. Agronomy. 2022 Sep;12(10):2347. 10.3390/agronomy12102347.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Supplementation of P-solubilizing purple nonsulfur bacteria, Rhodopseudomonas palustris improved soil fertility, P nutrient, growth, and yield of Cucumis melo L.
- Yield gap variation in rice cultivation in Indonesia
- Effects of co-inoculation of indole-3-acetic acid- and ammonia-producing bacteria on plant growth and nutrition, soil elements, and the relationships of soil microbiomes with soil physicochemical parameters
- Impact of mulching and planting time on spring-wheat (Triticum aestivum) growth: A combined field experiment and empirical modeling approach
- Morphological diversity, correlation studies, and multiple-traits selection for yield and yield components of local cowpea varieties
- Participatory on-farm evaluation of new orange-fleshed sweetpotato varieties in Southern Ethiopia
- Yield performance and stability analysis of three cultivars of Gayo Arabica coffee across six different environments
- Biology of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different types of plants feeds: Potency as a pest on various agricultural plants
- Antidiabetic activity of methanolic extract of Hibiscus sabdariffa Linn. fruit in alloxan-induced Swiss albino diabetic mice
- Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance
- Nicotinamide as a biostimulant improves soybean growth and yield
- Farmer’s willingness to accept the sustainable zoning-based organic farming development plan: A lesson from Sleman District, Indonesia
- Uncovering hidden determinants of millennial farmers’ intentions in running conservation agriculture: An application of the Norm Activation Model
- Mediating role of leadership and group capital between human capital component and sustainability of horticultural agribusiness institutions in Indonesia
- Biochar technology to increase cassava crop productivity: A study of sustainable agriculture on degraded land
- Effect of struvite on the growth of green beans on Mars and Moon regolith simulants
- UrbanAgriKG: A knowledge graph on urban agriculture and its embeddings
- Provision of loans and credit by cocoa buyers under non-price competition: Cocoa beans market in Ghana
- Effectiveness of micro-dosing of lime on selected chemical properties of soil in Banja District, North West, Ethiopia
- Effect of weather, nitrogen fertilizer, and biostimulators on the root size and yield components of Hordeum vulgare
- Effects of selected biostimulants on qualitative and quantitative parameters of nine cultivars of the genus Capsicum spp.
- Growth, yield, and secondary metabolite responses of three shallot cultivars at different watering intervals
- Design of drainage channel for effective use of land on fully mechanized sugarcane plantations: A case study at Bone Sugarcane Plantation
- Technical feasibility and economic benefit of combined shallot seedlings techniques in Indonesia
- Control of Meloidogyne javanica in banana by endophytic bacteria
- Comparison of important quality components of red-flesh kiwifruit (Actinidia chinensis) in different locations
- Efficiency of rice farming in flood-prone areas of East Java, Indonesia
- Comparative analysis of alpine agritourism in Trentino, Tyrol, and South Tyrol: Regional variations and prospects
- Detection of Fusarium spp. infection in potato (Solanum tuberosum L.) during postharvest storage through visible–near-infrared and shortwave–near-infrared reflectance spectroscopy
- Forage yield, seed, and forage qualitative traits evaluation by determining the optimal forage harvesting stage in dual-purpose cultivation in safflower varieties (Carthamus tinctorius L.)
- The influence of tourism on the development of urban space: Comparison in Hanoi, Danang, and Ho Chi Minh City
- Optimum intra-row spacing and clove size for the economical production of garlic (Allium sativum L.) in Northwestern Highlands of Ethiopia
- The role of organic rice farm income on farmer household welfare: Evidence from Yogyakarta, Indonesia
- Exploring innovative food in a developing country: Edible insects as a sustainable option
- Genotype by environment interaction and performance stability of common bean (Phaseolus vulgaris L.) cultivars grown in Dawuro zone, Southwestern Ethiopia
- Factors influencing green, environmentally-friendly consumer behaviour
- Factors affecting coffee farmers’ access to financial institutions: The case of Bandung Regency, Indonesia
- Morphological and yield trait-based evaluation and selection of chili (Capsicum annuum L.) genotypes suitable for both summer and winter seasons
- Sustainability analysis and decision-making strategy for swamp buffalo (Bubalus bubalis carabauesis) conservation in Jambi Province, Indonesia
- Understanding factors affecting rice purchasing decisions in Indonesia: Does rice brand matter?
- An implementation of an extended theory of planned behavior to investigate consumer behavior on hygiene sanitation-certified livestock food products
- Information technology adoption in Indonesia’s small-scale dairy farms
- Draft genome of a biological control agent against Bipolaris sorokiniana, the causal phytopathogen of spot blotch in wheat (Triticum turgidum L. subsp. durum): Bacillus inaquosorum TSO22
- Assessment of the recurrent mutagenesis efficacy of sesame crosses followed by isolation and evaluation of promising genetic resources for use in future breeding programs
- Fostering cocoa industry resilience: A collaborative approach to managing farm gate price fluctuations in West Sulawesi, Indonesia
- Field investigation of component failures for selected farm machinery used in small rice farming operations
- Near-infrared technology in agriculture: Rapid, simultaneous, and non-destructive determination of inner quality parameters on intact coffee beans
- The synergistic application of sucrose and various LED light exposures to enhance the in vitro growth of Stevia rebaudiana (Bertoni)
- Weather index-based agricultural insurance for flower farmers: Willingness to pay, sales, and profitability perspectives
- Meta-analysis of dietary Bacillus spp. on serum biochemical and antioxidant status and egg quality of laying hens
- Biochemical characterization of trypsin from Indonesian skipjack tuna (Katsuwonus pelamis) viscera
- Determination of C-factor for conventional cultivation and soil conservation technique used in hop gardens
- Empowering farmers: Unveiling the economic impacts of contract farming on red chilli farmers’ income in Magelang District, Indonesia
- Evaluating salt tolerance in fodder crops: A field experiment in the dry land
- Labor productivity of lowland rice (Oryza sativa L.) farmers in Central Java Province, Indonesia
- Cropping systems and production assessment in southern Myanmar: Informing strategic interventions
- The effect of biostimulants and red mud on the growth and yield of shallots in post-unlicensed gold mining soil
- Effects of dietary Adansonia digitata L. (baobab) seed meal on growth performance and carcass characteristics of broiler chickens: A systematic review and meta-analysis
- Analysis and structural characterization of the vid-pisco market
- Pseudomonas fluorescens SP007s enhances defense responses against the soybean bacterial pustule caused by Xanthomonas axonopodis pv. glycines
- A brief investigation on the prospective of co-composted biochar as a fertilizer for Zucchini plants cultivated in arid sandy soil
- Supply chain efficiency of red chilies in the production center of Sleman Indonesia based on performance measurement system
- Investment development path for developed economies: Is agriculture different?
- Power relations among actors in laying hen business in Indonesia: A MACTOR analysis
- High-throughput digital imaging and detection of morpho-physiological traits in tomato plants under drought
- Converting compression ignition engine to dual-fuel (diesel + CNG) engine and experimentally investigating its performance and emissions
- Structuration, risk management, and institutional dynamics in resolving palm oil conflicts
- Spacing strategies for enhancing drought resilience and yield in maize agriculture
- Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types
- Investigating Spodoptera spp. diversity, percentage of attack, and control strategies in the West Java, Indonesia, corn cultivation
- Yield stability of biofertilizer treatments to soybean in the rainy season based on the GGE biplot
- Evaluating agricultural yield and economic implications of varied irrigation depths on maize yield in semi-arid environments, at Birfarm, Upper Blue Nile, Ethiopia
- Chemometrics for mapping the spatial nitrate distribution on the leaf lamina of fenugreek grown under varying nitrogenous fertilizer doses
- Pomegranate peel ethanolic extract: A promising natural antioxidant, antimicrobial agent, and novel approach to mitigate rancidity in used edible oils
- Transformative learning and engagement with organic farming: Lessons learned from Indonesia
- Tourism in rural areas as a broader concept: Some insights from the Portuguese reality
- Assessment enhancing drought tolerance in henna (Lawsonia inermis L.) ecotypes through sodium nitroprusside foliar application
- Edible insects: A survey about perceptions regarding possible beneficial health effects and safety concerns among adult citizens from Portugal and Romania
- Phenological stages analysis in peach trees using electronic nose
- Harvest date and salicylic acid impact on peanut (Arachis hypogaea L.) properties under different humidity conditions
- Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential
- Use of different vegetation indices for the evaluation of the kinetics of the cherry tomato (Solanum lycopersicum var. cerasiforme) growth based on multispectral images by UAV
- First evidence of microplastic pollution in mangrove sediments and its ingestion by coral reef fish: Case study in Biawak Island, Indonesia
- Physical and textural properties and sensory acceptability of wheat bread partially incorporated with unripe non-commercial banana cultivars
- Cereibacter sphaeroides ST16 and ST26 were used to solubilize insoluble P forms to improve P uptake, growth, and yield of rice in acidic and extreme saline soil
- Avocado peel by-product in cattle diets and supplementation with oregano oil and effects on production, carcass, and meat quality
- Optimizing inorganic blended fertilizer application for the maximum grain yield and profitability of bread wheat and food barley in Dawuro Zone, Southwest Ethiopia
- The acceptance of social media as a channel of communication and livestock information for sheep farmers
- Adaptation of rice farmers to aging in Thailand
- Combined use of improved maize hybrids and nitrogen application increases grain yield of maize, under natural Striga hermonthica infestation
- From aquatic to terrestrial: An examination of plant diversity and ecological shifts
- Statistical modelling of a tractor tractive performance during ploughing operation on a tropical Alfisol
- Participation in artisanal diamond mining and food security: A case study of Kasai Oriental in DR Congo
- Assessment and multi-scenario simulation of ecosystem service values in Southwest China’s mountainous and hilly region
- Analysis of agricultural emissions and economic growth in Europe in search of ecological balance
- Bacillus thuringiensis strains with high insecticidal activity against insect larvae of the orders Coleoptera and Lepidoptera
- Technical efficiency of sugarcane farming in East Java, Indonesia: A bootstrap data envelopment analysis
- Comparison between mycobiota diversity and fungi and mycotoxin contamination of maize and wheat
- Evaluation of cultivation technology package and corn variety based on agronomy characters and leaf green indices
- Exploring the association between the consumption of beverages, fast foods, sweets, fats, and oils and the risk of gastric and pancreatic cancers: Findings from case–control study
- Phytochemical composition and insecticidal activity of Acokanthera oblongifolia (Hochst.) Benth & Hook.f. ex B.D.Jacks. extract on life span and biological aspects of Spodoptera littoralis (Biosd.)
- Land use management solutions in response to climate change: Case study in the central coastal areas of Vietnam
- Evaluation of coffee pulp as a feed ingredient for ruminants: A meta-analysis
- Interannual variations of normalized difference vegetation index and potential evapotranspiration and their relationship in the Baghdad area
- Harnessing synthetic microbial communities with nitrogen-fixing activity to promote rice growth
- Agronomic and economic benefits of rice–sweetpotato rotation in lowland rice cropping systems in Uganda
- Response of potato tuber as an effect of the N-fertilizer and paclobutrazol application in medium altitude
- Bridging the gap: The role of geographic proximity in enhancing seed sustainability in Bandung District
- Evaluation of Abrams curve in agricultural sector using the NARDL approach
- Challenges and opportunities for young farmers in the implementation of the Rural Development Program 2014–2020 of the Republic of Croatia
- Yield stability of ten common bean (Phaseolus vulgaris L.) genotypes at different sowing dates in Lubumbashi, South-East of DR Congo
- Effects of encapsulation and combining probiotics with different nitrate forms on methane emission and in vitro rumen fermentation characteristics
- Phytochemical analysis of Bienertia sinuspersici extract and its antioxidant and antimicrobial activities
- Evaluation of relative drought tolerance of grapevines by leaf fluorescence parameters
- Yield assessment of new streak-resistant topcross maize hybrids in Benin
- Improvement of cocoa powder properties through ultrasonic- and microwave-assisted alkalization
- Potential of ecoenzymes made from nutmeg (Myristica fragrans) leaf and pulp waste as bioinsecticides for Periplaneta americana
- Analysis of farm performance to realize the sustainability of organic cabbage vegetable farming in Getasan Semarang, Indonesia
- Revealing the influences of organic amendment-derived dissolved organic matter on growth and nutrient accumulation in lettuce seedlings (Lactuca sativa L.)
- Identification of viruses infecting sweetpotato (Ipomoea batatas Lam.) in Benin
- Assessing the soil physical and chemical properties of long-term pomelo orchard based on tree growth
- Investigating access and use of digital tools for agriculture among rural farmers: A case study of Nkomazi Municipality, South Africa
- Does sex influence the impact of dietary vitD3 and UVB light on performance parameters and welfare indicators of broilers?
- Design of intelligent sprayer control for an autonomous farming drone using a multiclass support vector machine
- Deciphering salt-responsive NB-ARC genes in rice transcriptomic data: A bioinformatics approach with gene expression validation
- Review Articles
- Impact of nematode infestation in livestock production and the role of natural feed additives – A review
- Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: A review
- Climate change and adaptive strategies on viticulture (Vitis spp.)
- The false tiger of almond, Monosteira unicostata (Hemiptera: Tingidae): Biology, ecology, and control methods
- A systematic review on potential analogy of phytobiomass and soil carbon evaluation methods: Ethiopia insights
- A review of storage temperature and relative humidity effects on shelf life and quality of mango (Mangifera indica L.) fruit and implications for nutrition insecurity in Ethiopia
- Green extraction of nutmeg (Myristica fragrans) phytochemicals: Prospective strategies and roadblocks
- Potential influence of nitrogen fertilizer rates on yield and yield components of carrot (Dacus carota L.) in Ethiopia: Systematic review
- Corn silk: A promising source of antimicrobial compounds for health and wellness
- State and contours of research on roselle (Hibiscus sabdariffa L.) in Africa
- The potential of phosphorus-solubilizing purple nonsulfur bacteria in agriculture: Present and future perspectives
- Minor millets: Processing techniques and their nutritional and health benefits
- Meta-analysis of reproductive performance of improved dairy cattle under Ethiopian environmental conditions
- Review on enhancing the efficiency of fertilizer utilization: Strategies for optimal nutrient management
- The nutritional, phytochemical composition, and utilisation of different parts of maize: A comparative analysis
- Motivations for farmers’ participation in agri-environmental scheme in the EU, literature review
- Evolution of climate-smart agriculture research: A science mapping exploration and network analysis
- Short Communications
- Music enrichment improves the behavior and leukocyte profile of dairy cattle
- Effect of pruning height and organic fertilization on the morphological and productive characteristics of Moringa oleifera Lam. in the Peruvian dry tropics
- Corrigendum
- Corrigendum to “Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance”
- Corrigendum to “Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types”
- Special issue: Smart Agriculture System for Sustainable Development: Methods and Practices
- Construction of a sustainable model to predict the moisture content of porang powder (Amorphophallus oncophyllus) based on pointed-scan visible near-infrared spectroscopy
- FruitVision: A deep learning based automatic fruit grading system
- Energy harvesting and ANFIS modeling of a PVDF/GO-ZNO piezoelectric nanogenerator on a UAV
- Effects of stress hormones on digestibility and performance in cattle: A review
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part II
- Assessment of omega-3 and omega-6 fatty acid profiles and ratio of omega-6/omega-3 of white eggs produced by laying hens fed diets enriched with omega-3 rich vegetable oil
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part II
- Special Issue on FCEM – International Web Conference on Food Choice & Eating Motivation: Message from the editor
- Fruit and vegetable consumption: Study involving Portuguese and French consumers
- Knowledge about consumption of milk: Study involving consumers from two European Countries – France and Portugal
Articles in the same Issue
- Regular Articles
- Supplementation of P-solubilizing purple nonsulfur bacteria, Rhodopseudomonas palustris improved soil fertility, P nutrient, growth, and yield of Cucumis melo L.
- Yield gap variation in rice cultivation in Indonesia
- Effects of co-inoculation of indole-3-acetic acid- and ammonia-producing bacteria on plant growth and nutrition, soil elements, and the relationships of soil microbiomes with soil physicochemical parameters
- Impact of mulching and planting time on spring-wheat (Triticum aestivum) growth: A combined field experiment and empirical modeling approach
- Morphological diversity, correlation studies, and multiple-traits selection for yield and yield components of local cowpea varieties
- Participatory on-farm evaluation of new orange-fleshed sweetpotato varieties in Southern Ethiopia
- Yield performance and stability analysis of three cultivars of Gayo Arabica coffee across six different environments
- Biology of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different types of plants feeds: Potency as a pest on various agricultural plants
- Antidiabetic activity of methanolic extract of Hibiscus sabdariffa Linn. fruit in alloxan-induced Swiss albino diabetic mice
- Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance
- Nicotinamide as a biostimulant improves soybean growth and yield
- Farmer’s willingness to accept the sustainable zoning-based organic farming development plan: A lesson from Sleman District, Indonesia
- Uncovering hidden determinants of millennial farmers’ intentions in running conservation agriculture: An application of the Norm Activation Model
- Mediating role of leadership and group capital between human capital component and sustainability of horticultural agribusiness institutions in Indonesia
- Biochar technology to increase cassava crop productivity: A study of sustainable agriculture on degraded land
- Effect of struvite on the growth of green beans on Mars and Moon regolith simulants
- UrbanAgriKG: A knowledge graph on urban agriculture and its embeddings
- Provision of loans and credit by cocoa buyers under non-price competition: Cocoa beans market in Ghana
- Effectiveness of micro-dosing of lime on selected chemical properties of soil in Banja District, North West, Ethiopia
- Effect of weather, nitrogen fertilizer, and biostimulators on the root size and yield components of Hordeum vulgare
- Effects of selected biostimulants on qualitative and quantitative parameters of nine cultivars of the genus Capsicum spp.
- Growth, yield, and secondary metabolite responses of three shallot cultivars at different watering intervals
- Design of drainage channel for effective use of land on fully mechanized sugarcane plantations: A case study at Bone Sugarcane Plantation
- Technical feasibility and economic benefit of combined shallot seedlings techniques in Indonesia
- Control of Meloidogyne javanica in banana by endophytic bacteria
- Comparison of important quality components of red-flesh kiwifruit (Actinidia chinensis) in different locations
- Efficiency of rice farming in flood-prone areas of East Java, Indonesia
- Comparative analysis of alpine agritourism in Trentino, Tyrol, and South Tyrol: Regional variations and prospects
- Detection of Fusarium spp. infection in potato (Solanum tuberosum L.) during postharvest storage through visible–near-infrared and shortwave–near-infrared reflectance spectroscopy
- Forage yield, seed, and forage qualitative traits evaluation by determining the optimal forage harvesting stage in dual-purpose cultivation in safflower varieties (Carthamus tinctorius L.)
- The influence of tourism on the development of urban space: Comparison in Hanoi, Danang, and Ho Chi Minh City
- Optimum intra-row spacing and clove size for the economical production of garlic (Allium sativum L.) in Northwestern Highlands of Ethiopia
- The role of organic rice farm income on farmer household welfare: Evidence from Yogyakarta, Indonesia
- Exploring innovative food in a developing country: Edible insects as a sustainable option
- Genotype by environment interaction and performance stability of common bean (Phaseolus vulgaris L.) cultivars grown in Dawuro zone, Southwestern Ethiopia
- Factors influencing green, environmentally-friendly consumer behaviour
- Factors affecting coffee farmers’ access to financial institutions: The case of Bandung Regency, Indonesia
- Morphological and yield trait-based evaluation and selection of chili (Capsicum annuum L.) genotypes suitable for both summer and winter seasons
- Sustainability analysis and decision-making strategy for swamp buffalo (Bubalus bubalis carabauesis) conservation in Jambi Province, Indonesia
- Understanding factors affecting rice purchasing decisions in Indonesia: Does rice brand matter?
- An implementation of an extended theory of planned behavior to investigate consumer behavior on hygiene sanitation-certified livestock food products
- Information technology adoption in Indonesia’s small-scale dairy farms
- Draft genome of a biological control agent against Bipolaris sorokiniana, the causal phytopathogen of spot blotch in wheat (Triticum turgidum L. subsp. durum): Bacillus inaquosorum TSO22
- Assessment of the recurrent mutagenesis efficacy of sesame crosses followed by isolation and evaluation of promising genetic resources for use in future breeding programs
- Fostering cocoa industry resilience: A collaborative approach to managing farm gate price fluctuations in West Sulawesi, Indonesia
- Field investigation of component failures for selected farm machinery used in small rice farming operations
- Near-infrared technology in agriculture: Rapid, simultaneous, and non-destructive determination of inner quality parameters on intact coffee beans
- The synergistic application of sucrose and various LED light exposures to enhance the in vitro growth of Stevia rebaudiana (Bertoni)
- Weather index-based agricultural insurance for flower farmers: Willingness to pay, sales, and profitability perspectives
- Meta-analysis of dietary Bacillus spp. on serum biochemical and antioxidant status and egg quality of laying hens
- Biochemical characterization of trypsin from Indonesian skipjack tuna (Katsuwonus pelamis) viscera
- Determination of C-factor for conventional cultivation and soil conservation technique used in hop gardens
- Empowering farmers: Unveiling the economic impacts of contract farming on red chilli farmers’ income in Magelang District, Indonesia
- Evaluating salt tolerance in fodder crops: A field experiment in the dry land
- Labor productivity of lowland rice (Oryza sativa L.) farmers in Central Java Province, Indonesia
- Cropping systems and production assessment in southern Myanmar: Informing strategic interventions
- The effect of biostimulants and red mud on the growth and yield of shallots in post-unlicensed gold mining soil
- Effects of dietary Adansonia digitata L. (baobab) seed meal on growth performance and carcass characteristics of broiler chickens: A systematic review and meta-analysis
- Analysis and structural characterization of the vid-pisco market
- Pseudomonas fluorescens SP007s enhances defense responses against the soybean bacterial pustule caused by Xanthomonas axonopodis pv. glycines
- A brief investigation on the prospective of co-composted biochar as a fertilizer for Zucchini plants cultivated in arid sandy soil
- Supply chain efficiency of red chilies in the production center of Sleman Indonesia based on performance measurement system
- Investment development path for developed economies: Is agriculture different?
- Power relations among actors in laying hen business in Indonesia: A MACTOR analysis
- High-throughput digital imaging and detection of morpho-physiological traits in tomato plants under drought
- Converting compression ignition engine to dual-fuel (diesel + CNG) engine and experimentally investigating its performance and emissions
- Structuration, risk management, and institutional dynamics in resolving palm oil conflicts
- Spacing strategies for enhancing drought resilience and yield in maize agriculture
- Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types
- Investigating Spodoptera spp. diversity, percentage of attack, and control strategies in the West Java, Indonesia, corn cultivation
- Yield stability of biofertilizer treatments to soybean in the rainy season based on the GGE biplot
- Evaluating agricultural yield and economic implications of varied irrigation depths on maize yield in semi-arid environments, at Birfarm, Upper Blue Nile, Ethiopia
- Chemometrics for mapping the spatial nitrate distribution on the leaf lamina of fenugreek grown under varying nitrogenous fertilizer doses
- Pomegranate peel ethanolic extract: A promising natural antioxidant, antimicrobial agent, and novel approach to mitigate rancidity in used edible oils
- Transformative learning and engagement with organic farming: Lessons learned from Indonesia
- Tourism in rural areas as a broader concept: Some insights from the Portuguese reality
- Assessment enhancing drought tolerance in henna (Lawsonia inermis L.) ecotypes through sodium nitroprusside foliar application
- Edible insects: A survey about perceptions regarding possible beneficial health effects and safety concerns among adult citizens from Portugal and Romania
- Phenological stages analysis in peach trees using electronic nose
- Harvest date and salicylic acid impact on peanut (Arachis hypogaea L.) properties under different humidity conditions
- Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential
- Use of different vegetation indices for the evaluation of the kinetics of the cherry tomato (Solanum lycopersicum var. cerasiforme) growth based on multispectral images by UAV
- First evidence of microplastic pollution in mangrove sediments and its ingestion by coral reef fish: Case study in Biawak Island, Indonesia
- Physical and textural properties and sensory acceptability of wheat bread partially incorporated with unripe non-commercial banana cultivars
- Cereibacter sphaeroides ST16 and ST26 were used to solubilize insoluble P forms to improve P uptake, growth, and yield of rice in acidic and extreme saline soil
- Avocado peel by-product in cattle diets and supplementation with oregano oil and effects on production, carcass, and meat quality
- Optimizing inorganic blended fertilizer application for the maximum grain yield and profitability of bread wheat and food barley in Dawuro Zone, Southwest Ethiopia
- The acceptance of social media as a channel of communication and livestock information for sheep farmers
- Adaptation of rice farmers to aging in Thailand
- Combined use of improved maize hybrids and nitrogen application increases grain yield of maize, under natural Striga hermonthica infestation
- From aquatic to terrestrial: An examination of plant diversity and ecological shifts
- Statistical modelling of a tractor tractive performance during ploughing operation on a tropical Alfisol
- Participation in artisanal diamond mining and food security: A case study of Kasai Oriental in DR Congo
- Assessment and multi-scenario simulation of ecosystem service values in Southwest China’s mountainous and hilly region
- Analysis of agricultural emissions and economic growth in Europe in search of ecological balance
- Bacillus thuringiensis strains with high insecticidal activity against insect larvae of the orders Coleoptera and Lepidoptera
- Technical efficiency of sugarcane farming in East Java, Indonesia: A bootstrap data envelopment analysis
- Comparison between mycobiota diversity and fungi and mycotoxin contamination of maize and wheat
- Evaluation of cultivation technology package and corn variety based on agronomy characters and leaf green indices
- Exploring the association between the consumption of beverages, fast foods, sweets, fats, and oils and the risk of gastric and pancreatic cancers: Findings from case–control study
- Phytochemical composition and insecticidal activity of Acokanthera oblongifolia (Hochst.) Benth & Hook.f. ex B.D.Jacks. extract on life span and biological aspects of Spodoptera littoralis (Biosd.)
- Land use management solutions in response to climate change: Case study in the central coastal areas of Vietnam
- Evaluation of coffee pulp as a feed ingredient for ruminants: A meta-analysis
- Interannual variations of normalized difference vegetation index and potential evapotranspiration and their relationship in the Baghdad area
- Harnessing synthetic microbial communities with nitrogen-fixing activity to promote rice growth
- Agronomic and economic benefits of rice–sweetpotato rotation in lowland rice cropping systems in Uganda
- Response of potato tuber as an effect of the N-fertilizer and paclobutrazol application in medium altitude
- Bridging the gap: The role of geographic proximity in enhancing seed sustainability in Bandung District
- Evaluation of Abrams curve in agricultural sector using the NARDL approach
- Challenges and opportunities for young farmers in the implementation of the Rural Development Program 2014–2020 of the Republic of Croatia
- Yield stability of ten common bean (Phaseolus vulgaris L.) genotypes at different sowing dates in Lubumbashi, South-East of DR Congo
- Effects of encapsulation and combining probiotics with different nitrate forms on methane emission and in vitro rumen fermentation characteristics
- Phytochemical analysis of Bienertia sinuspersici extract and its antioxidant and antimicrobial activities
- Evaluation of relative drought tolerance of grapevines by leaf fluorescence parameters
- Yield assessment of new streak-resistant topcross maize hybrids in Benin
- Improvement of cocoa powder properties through ultrasonic- and microwave-assisted alkalization
- Potential of ecoenzymes made from nutmeg (Myristica fragrans) leaf and pulp waste as bioinsecticides for Periplaneta americana
- Analysis of farm performance to realize the sustainability of organic cabbage vegetable farming in Getasan Semarang, Indonesia
- Revealing the influences of organic amendment-derived dissolved organic matter on growth and nutrient accumulation in lettuce seedlings (Lactuca sativa L.)
- Identification of viruses infecting sweetpotato (Ipomoea batatas Lam.) in Benin
- Assessing the soil physical and chemical properties of long-term pomelo orchard based on tree growth
- Investigating access and use of digital tools for agriculture among rural farmers: A case study of Nkomazi Municipality, South Africa
- Does sex influence the impact of dietary vitD3 and UVB light on performance parameters and welfare indicators of broilers?
- Design of intelligent sprayer control for an autonomous farming drone using a multiclass support vector machine
- Deciphering salt-responsive NB-ARC genes in rice transcriptomic data: A bioinformatics approach with gene expression validation
- Review Articles
- Impact of nematode infestation in livestock production and the role of natural feed additives – A review
- Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: A review
- Climate change and adaptive strategies on viticulture (Vitis spp.)
- The false tiger of almond, Monosteira unicostata (Hemiptera: Tingidae): Biology, ecology, and control methods
- A systematic review on potential analogy of phytobiomass and soil carbon evaluation methods: Ethiopia insights
- A review of storage temperature and relative humidity effects on shelf life and quality of mango (Mangifera indica L.) fruit and implications for nutrition insecurity in Ethiopia
- Green extraction of nutmeg (Myristica fragrans) phytochemicals: Prospective strategies and roadblocks
- Potential influence of nitrogen fertilizer rates on yield and yield components of carrot (Dacus carota L.) in Ethiopia: Systematic review
- Corn silk: A promising source of antimicrobial compounds for health and wellness
- State and contours of research on roselle (Hibiscus sabdariffa L.) in Africa
- The potential of phosphorus-solubilizing purple nonsulfur bacteria in agriculture: Present and future perspectives
- Minor millets: Processing techniques and their nutritional and health benefits
- Meta-analysis of reproductive performance of improved dairy cattle under Ethiopian environmental conditions
- Review on enhancing the efficiency of fertilizer utilization: Strategies for optimal nutrient management
- The nutritional, phytochemical composition, and utilisation of different parts of maize: A comparative analysis
- Motivations for farmers’ participation in agri-environmental scheme in the EU, literature review
- Evolution of climate-smart agriculture research: A science mapping exploration and network analysis
- Short Communications
- Music enrichment improves the behavior and leukocyte profile of dairy cattle
- Effect of pruning height and organic fertilization on the morphological and productive characteristics of Moringa oleifera Lam. in the Peruvian dry tropics
- Corrigendum
- Corrigendum to “Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance”
- Corrigendum to “Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types”
- Special issue: Smart Agriculture System for Sustainable Development: Methods and Practices
- Construction of a sustainable model to predict the moisture content of porang powder (Amorphophallus oncophyllus) based on pointed-scan visible near-infrared spectroscopy
- FruitVision: A deep learning based automatic fruit grading system
- Energy harvesting and ANFIS modeling of a PVDF/GO-ZNO piezoelectric nanogenerator on a UAV
- Effects of stress hormones on digestibility and performance in cattle: A review
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part II
- Assessment of omega-3 and omega-6 fatty acid profiles and ratio of omega-6/omega-3 of white eggs produced by laying hens fed diets enriched with omega-3 rich vegetable oil
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part II
- Special Issue on FCEM – International Web Conference on Food Choice & Eating Motivation: Message from the editor
- Fruit and vegetable consumption: Study involving Portuguese and French consumers
- Knowledge about consumption of milk: Study involving consumers from two European Countries – France and Portugal

