Abstract
C25H15NO3, triclinic, P1̄ (no. 2), a = 8.4899(3) Å, b = 10.1383(4) Å, c = 11.3806(5) Å, α = 78.373(1)°, β = 72.020(1)°, γ = 73.381(1)°, V = 885.89(6) Å3, Z = 2, R gt (F) = 0.0439, wR ref (F2) = 0.1290, T = 296.15 K.
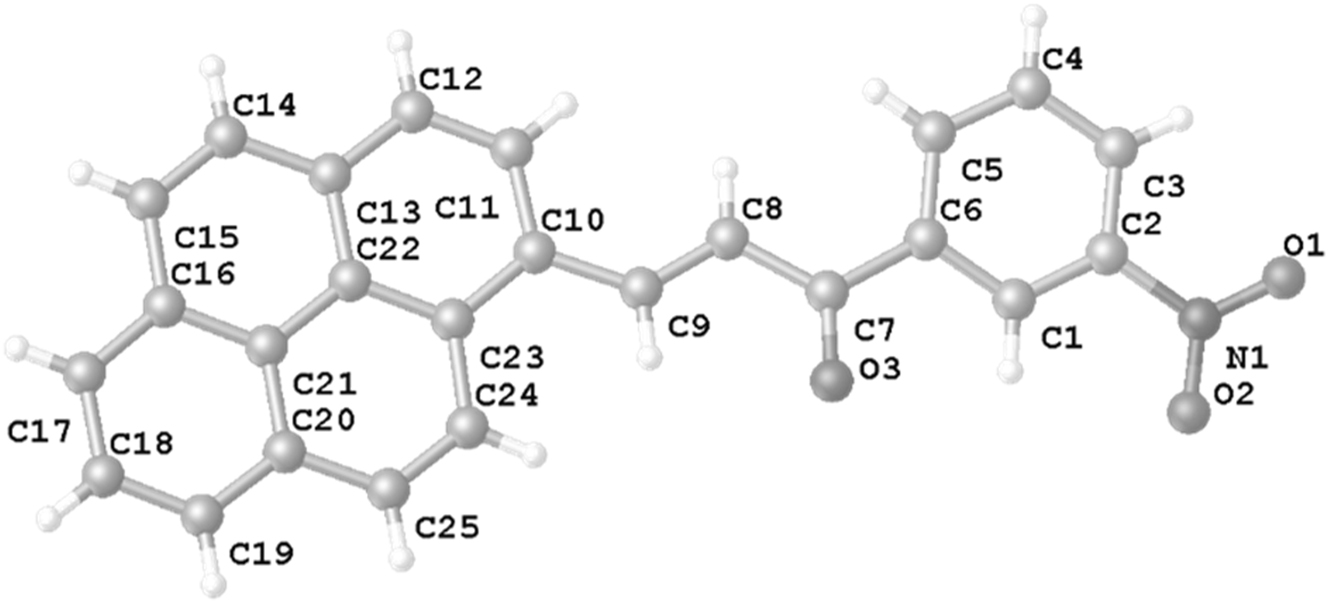
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange block |
| Size: | 0.16 × 0.16 × 0.12 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 0.09 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Bruker APEX-II, φ and ω 26.4°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 25692, 3611, 0.038 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2790 |
| N(param)refined: | 263 |
| Programs: | Olex2, 1 , 2 SHELX, 3 PLATON 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.82079 (18) | −0.09534 (14) | 0.82596 (14) | 0.0484 (4) |
| H1 | 0.88114 (18) | −0.02805 (14) | 0.78625 (14) | 0.0581 (4)* |
| C2 | 0.90100 (18) | −0.22121 (15) | 0.87785 (14) | 0.0489 (4) |
| C3 | 0.8171 (2) | −0.32418 (15) | 0.93693 (14) | 0.0530 (4) |
| H3 | 0.8743 (2) | −0.40886 (15) | 0.97073 (14) | 0.0636 (5)* |
| C4 | 0.6468 (2) | −0.29854 (15) | 0.94458 (14) | 0.0556 (4) |
| H4 | 0.5876 (2) | −0.36661 (15) | 0.98418 (14) | 0.0667 (5)* |
| C5 | 0.56208 (19) | −0.17244 (15) | 0.89399 (14) | 0.0505 (4) |
| H5 | 0.44639 (19) | −0.15632 (15) | 0.90054 (14) | 0.0606 (4)* |
| C6 | 0.64827 (18) | −0.06983 (14) | 0.83358 (13) | 0.0456 (3) |
| C7 | 0.56828 (19) | 0.06617 (14) | 0.77011 (14) | 0.0502 (4) |
| C8 | 0.38248 (19) | 0.11314 (15) | 0.79359 (14) | 0.0524 (4) |
| H8 | 0.31296 (19) | 0.05646 (15) | 0.84480 (14) | 0.0629 (4)* |
| C9 | 0.31403 (19) | 0.23666 (15) | 0.74157 (14) | 0.0503 (4) |
| H9 | 0.39159 (19) | 0.28787 (15) | 0.69282 (14) | 0.0604 (4)* |
| C10 | 0.13601 (18) | 0.30429 (14) | 0.74915 (13) | 0.0460 (3) |
| C11 | 0.0070 (2) | 0.23634 (15) | 0.81603 (14) | 0.0539 (4) |
| H11 | 0.0357 (2) | 0.14931 (15) | 0.86023 (14) | 0.0646 (5)* |
| C12 | −0.16074 (19) | 0.29460 (16) | 0.81814 (15) | 0.0553 (4) |
| H12 | −0.24310 (19) | 0.24659 (16) | 0.86393 (15) | 0.0664 (5)* |
| C13 | −0.21009 (18) | 0.42467 (15) | 0.75282 (13) | 0.0481 (3) |
| C14 | −0.38354 (19) | 0.48695 (17) | 0.74993 (15) | 0.0566 (4) |
| H14 | −0.46712 (19) | 0.43890 (17) | 0.79223 (15) | 0.0679 (5)* |
| C15 | −0.42811 (19) | 0.61322 (17) | 0.68747 (15) | 0.0584 (4) |
| H15 | −0.54193 (19) | 0.65075 (17) | 0.68812 (15) | 0.0700 (5)* |
| C16 | −0.30510 (18) | 0.69073 (15) | 0.62041 (14) | 0.0503 (4) |
| C17 | −0.3478 (2) | 0.82252 (18) | 0.55483 (16) | 0.0617 (4) |
| H17 | −0.4609 (2) | 0.86207 (18) | 0.55453 (16) | 0.0741 (5)* |
| C18 | −0.2259 (2) | 0.89449 (17) | 0.49098 (16) | 0.0643 (5) |
| H18 | −0.2574 (2) | 0.98193 (17) | 0.44825 (16) | 0.0772 (5)* |
| C19 | −0.0572 (2) | 0.83832 (16) | 0.48963 (15) | 0.0562 (4) |
| H19 | 0.0242 (2) | 0.88800 (16) | 0.44571 (15) | 0.0674 (5)* |
| C20 | −0.00734 (18) | 0.70739 (14) | 0.55370 (13) | 0.0464 (3) |
| C21 | −0.13190 (17) | 0.63218 (14) | 0.62037 (12) | 0.0434 (3) |
| C22 | −0.08343 (17) | 0.49822 (14) | 0.68613 (12) | 0.0420 (3) |
| C23 | 0.09093 (17) | 0.43875 (14) | 0.68442 (12) | 0.0426 (3) |
| C24 | 0.21231 (18) | 0.51862 (15) | 0.61550 (14) | 0.0492 (4) |
| H24 | 0.32675 (18) | 0.48167 (15) | 0.61317 (14) | 0.0591 (4)* |
| C25 | 0.16610 (18) | 0.64542 (15) | 0.55402 (14) | 0.0508 (4) |
| H25 | 0.24915 (18) | 0.69369 (15) | 0.51079 (14) | 0.0609 (4)* |
| N1 | 1.08375 (17) | −0.24715 (14) | 0.86821 (14) | 0.0632 (4) |
| O1 | 1.16062 (16) | −0.36356 (13) | 0.89792 (13) | 0.0801 (4) |
| O2 | 1.15100 (17) | −0.15056 (15) | 0.83108 (19) | 0.1084 (6) |
| O3 | 0.66127 (14) | 0.13690 (11) | 0.69929 (13) | 0.0770 (4) |
1 Source of materials
Equimolar amounts of 1-pyrencarboxaldehyde and 3-nitroacetophenone were carried out under reflux for 8 h in ethanol with two drops of piperidine as a catalyst. 5 The solid product precipitates in the reaction medium, and then is filtered under vacuum and washed with hot ethanol to eliminate all traces of starting reagents. The product is a yellow solid. To obtain single crystals, the solid product was placed in hot DMF and left to crystallize for two weeks, or until orange blocks started to emerge (E)-1-((3)-nitrophenyl)-3-(pyren-1-yl)prop-2-en-1-one. Yield 85 %. 1H NMR (400 MHz, DMSO‑d6) δ 8.97 (d, J = 15.3 Hz, 1H), 8.92 (t, J = 2.0 Hz, 1H), 8.89 (d, J = 8.2 Hz, 1H), 8.71 (dt, J = 7.9, 1.3 Hz, 1H), 8.65 (d, J = 9.4 Hz, 1H), 8.53–8.48 (m, 1H),8.42–8.22 (m, 7H), 8.14 (t, J = 7.6 Hz, 1H), 7.91 (t, J = 8.0 Hz, 1H). 13C NMR (101 MHz, DMSO) δ 187.80, 148.72, 141.52, 139.35, 135.27, 133.27, 131.29, 131.09, 130.63, 130.41, 129.51, 129.42, 128.33, 127.86, 127.82, 127.18, 126.86, 126.62, 125.74, 125.73, 124.55, 124.19, 123.79, 123.39, 122.76.
2 Experimental details
Using Olex2, 1 with the olex2.solve 2 using Charge Flipping and refined with the SHELXL 3 the structure was solved. SADABS-2016/2 (Bruker, 2016/2) was used for absorption correction. Interactions were calculated using Platon. 4
H atoms were finally included in their calculated positions and treated as riding on their parent atom with constrained thermal parameters as Uiso(H) = 1.2 Ueq(C), the constraint distances of C–H was 0.93 Å.
3 Comment
Chalcones are organic compounds found in plants and are related to the flavonoid family, structurally present two aromatic fragments linked through a double bond conjugated to a carbonyl, 6 these compounds are vastly studied and synthesized due to their chemical modifications that allow preparing of multiple derivatives including heterocyclic compounds. 7 Some chalcones show biological properties such as analgesic, 8 anti-inflammatory, 9 antiplatelet, 10 antioxidant 11 for example. By other hand, pyrenes are known for optical uses, and pyrene chalcones are molecules that can be transformed into other structures with interesting photo physicochemical properties. 12 In this structure there are two crystallographically independent molecules in the asymmetric unit. The N–O bond lengths in the nitro group range from 1.2115(18) to 1.2116(17) Å. The angle between O–N–O in these structures is 122.76(15)°, O–N–C range between 118.91(14)° to 118.33(13)°, similar value to reported by us in Barrientos, et al. 13 , 14 , 15 There are no classical hydrogen bonds in the crystal packing, 4 it only exhibits intermolecular C–H⋯O interactions with distances of 2.376(2)–2.525(2) Å between the donor and acceptor atoms. 16 The crystal structure exhibits N–O⋯π interactions N(1)–O(1)⋯Cg(2) = 3.8000(15) Å and N(1)–O(1)⋯Cg(5) = 3.8326(15) Å.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: ANID Fondecyt Regular 1210899 and FONDEQUIP EQM200138 for D8 Venture diffractometer.
References
1. Bourhis, L. J.; Dolomanov, O. V.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment – Olex2 Dissected. Acta Crystallogr. 2015, A71, 59–75; https://doi.org/10.1107/s2053273314022207.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Spek, A. L. Single-crystal Structure Validation with the Program Platon. J. Appl. Crystallogr. 2003, 36, 7–13; https://doi.org/10.1107/s0021889802022112.Search in Google Scholar
5. Karuppusamy, A.; Sharma, A.; Justin Thomas, K. R.; Kannan, P. Experimental and Theoretical Investigations on Chalcones Containing Pyrene. J. Mol. Struct. 2022, 1268, 133532; https://doi.org/10.1016/j.molstruc.2022.133532.Search in Google Scholar
6. Alam, M. S.; Rahman, S. M.; Lee, D.-U. Synthesis, Biological Evaluation, Quantitative–SAR and Docking Studies of Novel Chalcone Derivatives as Antibacterial and Antioxidant Agents. Chem. Pap. 2015, 69, 1118–1129; https://doi.org/10.1515/chempap-2015-0113.Search in Google Scholar
7. Rammohan, A.; Reddy, J. S.; Sravya, G.; Rao, C. N.; Zyryanov, G. V. Chalcone Synthesis, Properties and Medicinal Applications: a Review. Environ. Chem. Lett. 2020, 18, 433–458; https://doi.org/10.1007/s10311-019-00959-w.Search in Google Scholar
8. Lakshminarayanan, B.; Kannappan, N.; Subburaju, T. Synthesis and Biological Evaluation of Novel Chalcones with Methanesulfonyl End as Potent Analgesic and Anti-inflammatory Agents. Int. J. Pharmaceut. Res. Biosci 2020, 11, 4974–4981.Search in Google Scholar
9. Ibrahim, T. S.; Moustafa, A. H.; Almalki, A. J.; Allam, R. M.; Althagafi, A.; Md, S.; Mohamed, M. F. Novel Chalcone/aryl Carboximidamide Hybrids as Potent Anti-inflammatory via Inhibition of Prostaglandin E2 and Inducible NO Synthase Activities: Design, Synthesis, Molecular Docking Studies and ADMET Prediction. J. Enzym. Inhib. Med. Chem. 2021, 36, 1067–1078; https://doi.org/10.1080/14756366.2021.1929201.Search in Google Scholar PubMed PubMed Central
10. Fakhrudin, N.; Pertiwi, K. K.; Takubessi, M. I.; Susiani, E. F.; Nurrochmad, A.; Widyarini, S.; Sudarmanto, A.; Nugroho, A. A.; Wahyuono, S. A Geranylated Chalcone with Antiplatelet Activity from the Leaves of Breadfruit (Artocarpus altilis). Pharmacia 2020, 67, 173–180; https://doi.org/10.3897/pharmacia.67.e56788.Search in Google Scholar
11. Al Zahrani, N. A.; El-Shishtawy, R. M.; Elaasser, M. M.; Asiri, A. M. Synthesis of Novel Chalcone-Based Phenothiazine Derivatives as Antioxidant and Anticancer Agents. Molecules 2020, 25, 4566; https://doi.org/10.3390/molecules25194566.Search in Google Scholar PubMed PubMed Central
12. Karuppusamy, A.; Vandana, T.; Kannan, P. Pyrene Based Chalcone Materials as Solid State Luminogens with Aggregation-Induced Enhanced Emission Properties. J. Photochem. Photobiol., A 2017, 345, 11–20; https://doi.org/10.1016/j.jphotochem.2017.05.026.Search in Google Scholar
13. Barrientos, C.; Barahona, P.; Guevara, J. L.; Squella, J. A.; Moris, S. The Crystal Structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 1213–1214; https://doi.org/10.1515/ncrs-2019-0340.Search in Google Scholar
14. Barrientos, C.; Squella, J. A.; Moris, S. The Crystal Structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 459–461; https://doi.org/10.1515/ncrs-2023-0034.Search in Google Scholar
15. Barrientos, C.; Galdámez, A.; Moris, S. The Crystal Structure of 5-Nitronaphthoquinone, C10H5NO4. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 787–789; https://doi.org/10.1515/ncrs-2023-0211.Search in Google Scholar
16. Moris, S.; Galdámez, A.; Jara, P.; Saitz-Barria, C. Synthesis of Novel p-tert–Butylcalix[4]arene Derivative: Structural Characterization of a Methanol Inclusion Compound. Crystals 2016, 6, 114; https://doi.org/10.3390/cryst6090114.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of N-(3-bromo-4-fluorophenyl)-N′-hydroxy-4-{[2-(4-methylphenyl)ethyl]amino}-1,2,5-oxadiazole-3-carboximidamide, C18H17BrFN5O2
- Synthesis and crystal structure of ethyl (2S,4aS,6aS,6bR,8aR,12aS,12bR,14bR,E)-10-(((3,4-dichlorobenzyl)oxy)imino)-2,4a,6a,6b,9,9,12a-heptamethyl-13-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-2-carboxylate
- The crystal structure of pyrazole nitrate
- Crystal structure of tetramethyl-bis(μ2-2-(2-hydroxy-3-methoxybenzylidene)-1-(6-(2-(2-hydroxy-3-methoxybenzylidene)hydrazine-1-carbonyl)picolinoyl)hydrazin-1-ido-κ4O,N,O′:O′)ditin(II) ─ ethanol (1/2), C54H62N10O14Sn2
- Crystal structure of catena-poly[μ3-iodido-(4-bromopyridine-κ1N)copper(I)], C5H4BrNCuI
- The crystal structure of cyclopentadienyl Co–P–C complexes by benzylideneacetone addition, C38H38CoO2P
- Synthesis and crystal structure of-(3S,10S,13S,17S)-N-(2-methoxyphenyl)-10,13-dimethyl-17-((R)-1-(phenylamino)ethyl)hexadecahydro-1H-cyclopenta[α]phenanthren-3-amine, C34H48N2O
- The crystal structure of (E)-3-((E)-3-(4-ethoxy-3-methoxyphenyl)-1-hydroxyallylidene) chroman-2,4-dione, C21H18O6
- The crystal structure of trans–L/D-[bis-(2-methyl-8-hydroxyquinoline-κ2 N,O) bis-(1,3,5-triaza-7-phosphaadamantane-κ2 P)cobalt(III)] tetrafluoroborate
- Crystal structure of 9-chloro-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C14H15ClN4
- Crystal structure of 7-(diethylamino)-3-(benzoyl)-2 H -chromen-2-one, C20H19NO3
- The crystal structure of 4–bromo-3,5-dinitropyrazole
- Crystal structure of 8-hydroxy-3,5,8a-trimethyl-7,8,8a,9-tetrahydronaphtho[2,3-b]furan-4,6-dione, C15H16O4
- Crystal structure of 5-hydroxy-3,5,8a-trimethyl-4a,5,6,7,8a,9-hexahydronaphtho[2,3-b]furan-4,8-dione, C15H18O4
- Synthesis and crystal structure of-(3S,10S,13S,17S)-N-(2-methoxyphenyl)-10,13-dimethyl-17-((R)-1-(p-tolylamino)ethyl)hexadecahydro-1H-cyclopenta[α]phenanthren-3-amine, C35H50N2O
- The crystal structure of catena-poly((μ2-1,3,5-tri(1H- imidazol-1-yl)benzene κ2N:N′)-bis(4-hydroxbenzoato-κ1O)-zinc(II) monohydrate), C29H24N6O7Zn
- Crystal structure of 2-(benzo[d]oxazol-2-yl)acetonitrile, C9H6N2O
- Crystal structure of 1,3-dihydroxy-6,8-dimethoxy-2-(6-methyltetrahydro-2Hpyran-2-yl)-4a,9a-dihydroanthracene-9,10-dione, C22H22O7
- The crystal structure of the double salt potassium 1-methylpiperazine-1,4-di-ium trinitrate, C5H14KN5O9
- Crystal structure of 5′-hydroxy-6′-methoxy-1′-methyl-2′,3′,8′,8a′-tetrahydro-1′H-spiro[cyclohexane-1,7′-cyclopenta[ij]isoquinoline]-2,5-dien-4-one, C18H19NO3
- The crystal structure of 1,1′-(2,3,5,6-tetramethylpyrazine-1,4-diyl)bis(ethan-1-one), C12H18N2O2
- Crystal structure of [μ2-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ2O:O′)] bis(μ2-4,4′-trimethylenedipyridyl-κ2N:N′)disilver(I), C18H24AgN3O4S
- Crystal structure of bis ((1-((E)-((4-methoxyphenyl)imino)methyl)naphthalen-2-yl)oxy) copper(II), C36H28CuN2O4
- Synthesis and crystal structure of 6,6′-((1E,11E)-5,8-dioxa-2,11-diazadodeca-1,11-diene-1,12-diyl) bis(2,4-di-tert-butylphenol), C36H56N2O4
- The crystal structure of barium hexahydroxidoiridate(IV) dihydroxide, Ba2[Ir(OH)6](OH)2
- Crystal structure of cinnamoyl ferrocene, C19H16FeO
- Crystal structure of (E)-3-(4-butoxyphenyl)acryloylferrocene, C23H24FeO2
- Crystal structure of 7-(dimethylamino)-2-hydroxy-2-(trifluoromethyl)-2H-chromene-3-ethyl carboxylate, C15H16F3NO4
- The crystal structure of 1-phenylethan-1-aminium 4-hydroxy-3,5-dimethoxybenzoate C17H21NO5
- The crystal structure of 1,3,5-trichloro-2-nitrobenzene
- The crystal structure of tris(μ2-bromido)-bis(η6-p-cymene)-diosmium(II) tetrafluoroborate, C20H28BBr3F4Os2
- Crystal structure of new barium lithium manganese fluorides: Ba14Li1.87Mn14.13F68 with a Jarlite–related structure
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium chloride, C25H21ClFP
- The crystal structure of calcitriol–chloroform (1/1), C27H44O3⋅CHCl3
- The crystal structure of (E)-1-((3)-nitrophenyl)pyren-3-(pyren-1-yl)prop-2-en-1-one, C25H15NO3
- Crystal structure of (E)-2-hydroxy-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H16N2O3
- Crystal structure of (E)-(3-(thiophen-2-yl)acryloyl)ferrocene, C17H14FeOS
- Crystal structure of (E)-(3-(furan-2-yl)acryloyl)ferrocene, C17H14FeO2
- Synthesis and crystal structure poly[diaqua(μ3-3-(((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl) methyl)ammonio)propanoate-κ3 O:O′:O″) sodium(I)] monohydrate, C20H24NNaO12S
- Crystal structure of 9-methoxy-4-(2-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine C19H18N4O2
- Synthesis and crystal structure of 4-(difluoromethyl)-1-methyl-N-(pyridin-3-yl)-1H-pyrazole-3-carboxamide hydrate, C11H12F2N4O2
- The crystal structure of caesalfurfuric acid B, C22H32O4
- The crystal structure of 2-bromo-2-(5-bromo-2-methyl-4-nitro-1H-imidazol-1-yl)-1-phenylethanone, C12H9Br2N3O3
- The crystal structure of bis{chlorido-[μ2-(1-oxidopyridin-2-yl)(pyridin-2-yl)amido-κ3 O,N, N′]copper(II)}, C20H16Cl2Cu2N6O2
- The crystal structure of 3-amino-2-formyl-1-phenyl-9,10-dihydrophenanthrene-4-carbonitrile, C22H16N2O
- The crystal structure of 1,1′-(2,5-dimethylpyrazine-1,4-diyl)bis(ethan-1-one), C10H14N2O2
- Crystal structure of 5′-(9-phenyl-9H-carbazol-3-yl)-[2,2′-bithiophene]-5-carbaldehyde, C27H17NOS2
- The crystal structure of the double salt dipyridin-1-ium bromide tribromide
- Crystal structure of (E)-(3-(3-methylthiophen-2-yl)acryloyl)ferrocene, C18H16FeOS
- Crystal structure of (E)-(3-(4-phenoxyphenyl)acryloyl)ferrocene, C25H20FeO2
- Crystal structure of (E)-(3-(3,4-dimethylphenyl)acryloyl)ferrocene, C21H20FeO
- Crystal structure of [(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N‴)tetracyanidodiplatinum(II)] dimethyl sulfoxide solvate, C18H36N8O2Pt2S2
- Crystal structure of (4-ethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO2P
- Crystal structure of (1-naphthalen-1-yl-methyl)triphenylphosphonium chloride ethanol solvate, C31H30ClOP
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)platinum(II) bis[tribromido(dimethyl sulfoxide-κS)platinate(II)], C14H36Br6N4O2Pt3S2
- Crystal structure of (2-methylbenzyl)triphenylphosphonium chloride ethanol solvate, C28H30ClOP
- Crystal structure of bis(η2, σ1-8-methoxycyclooct-4-enyl)(μ2-1,4,8,11-tetraazacyclotetradecane-κ4 N, N′, N″, N‴)diplatinum(II) dibromide, C28H54Br2N4O2Pt2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)palladium(II) tetrabromidopalladate(II), C10H24Br4N4Pd2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)palladium(II) bis[trichlorido(dimethyl sulfoxide-κS)platinate(II)], C14H36Cl6N4O2PdPt2S2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)palladium(II) tetraiodidopalladate(II), C10H24I4N4Pd2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of N-(3-bromo-4-fluorophenyl)-N′-hydroxy-4-{[2-(4-methylphenyl)ethyl]amino}-1,2,5-oxadiazole-3-carboximidamide, C18H17BrFN5O2
- Synthesis and crystal structure of ethyl (2S,4aS,6aS,6bR,8aR,12aS,12bR,14bR,E)-10-(((3,4-dichlorobenzyl)oxy)imino)-2,4a,6a,6b,9,9,12a-heptamethyl-13-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-2-carboxylate
- The crystal structure of pyrazole nitrate
- Crystal structure of tetramethyl-bis(μ2-2-(2-hydroxy-3-methoxybenzylidene)-1-(6-(2-(2-hydroxy-3-methoxybenzylidene)hydrazine-1-carbonyl)picolinoyl)hydrazin-1-ido-κ4O,N,O′:O′)ditin(II) ─ ethanol (1/2), C54H62N10O14Sn2
- Crystal structure of catena-poly[μ3-iodido-(4-bromopyridine-κ1N)copper(I)], C5H4BrNCuI
- The crystal structure of cyclopentadienyl Co–P–C complexes by benzylideneacetone addition, C38H38CoO2P
- Synthesis and crystal structure of-(3S,10S,13S,17S)-N-(2-methoxyphenyl)-10,13-dimethyl-17-((R)-1-(phenylamino)ethyl)hexadecahydro-1H-cyclopenta[α]phenanthren-3-amine, C34H48N2O
- The crystal structure of (E)-3-((E)-3-(4-ethoxy-3-methoxyphenyl)-1-hydroxyallylidene) chroman-2,4-dione, C21H18O6
- The crystal structure of trans–L/D-[bis-(2-methyl-8-hydroxyquinoline-κ2 N,O) bis-(1,3,5-triaza-7-phosphaadamantane-κ2 P)cobalt(III)] tetrafluoroborate
- Crystal structure of 9-chloro-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C14H15ClN4
- Crystal structure of 7-(diethylamino)-3-(benzoyl)-2 H -chromen-2-one, C20H19NO3
- The crystal structure of 4–bromo-3,5-dinitropyrazole
- Crystal structure of 8-hydroxy-3,5,8a-trimethyl-7,8,8a,9-tetrahydronaphtho[2,3-b]furan-4,6-dione, C15H16O4
- Crystal structure of 5-hydroxy-3,5,8a-trimethyl-4a,5,6,7,8a,9-hexahydronaphtho[2,3-b]furan-4,8-dione, C15H18O4
- Synthesis and crystal structure of-(3S,10S,13S,17S)-N-(2-methoxyphenyl)-10,13-dimethyl-17-((R)-1-(p-tolylamino)ethyl)hexadecahydro-1H-cyclopenta[α]phenanthren-3-amine, C35H50N2O
- The crystal structure of catena-poly((μ2-1,3,5-tri(1H- imidazol-1-yl)benzene κ2N:N′)-bis(4-hydroxbenzoato-κ1O)-zinc(II) monohydrate), C29H24N6O7Zn
- Crystal structure of 2-(benzo[d]oxazol-2-yl)acetonitrile, C9H6N2O
- Crystal structure of 1,3-dihydroxy-6,8-dimethoxy-2-(6-methyltetrahydro-2Hpyran-2-yl)-4a,9a-dihydroanthracene-9,10-dione, C22H22O7
- The crystal structure of the double salt potassium 1-methylpiperazine-1,4-di-ium trinitrate, C5H14KN5O9
- Crystal structure of 5′-hydroxy-6′-methoxy-1′-methyl-2′,3′,8′,8a′-tetrahydro-1′H-spiro[cyclohexane-1,7′-cyclopenta[ij]isoquinoline]-2,5-dien-4-one, C18H19NO3
- The crystal structure of 1,1′-(2,3,5,6-tetramethylpyrazine-1,4-diyl)bis(ethan-1-one), C12H18N2O2
- Crystal structure of [μ2-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ2O:O′)] bis(μ2-4,4′-trimethylenedipyridyl-κ2N:N′)disilver(I), C18H24AgN3O4S
- Crystal structure of bis ((1-((E)-((4-methoxyphenyl)imino)methyl)naphthalen-2-yl)oxy) copper(II), C36H28CuN2O4
- Synthesis and crystal structure of 6,6′-((1E,11E)-5,8-dioxa-2,11-diazadodeca-1,11-diene-1,12-diyl) bis(2,4-di-tert-butylphenol), C36H56N2O4
- The crystal structure of barium hexahydroxidoiridate(IV) dihydroxide, Ba2[Ir(OH)6](OH)2
- Crystal structure of cinnamoyl ferrocene, C19H16FeO
- Crystal structure of (E)-3-(4-butoxyphenyl)acryloylferrocene, C23H24FeO2
- Crystal structure of 7-(dimethylamino)-2-hydroxy-2-(trifluoromethyl)-2H-chromene-3-ethyl carboxylate, C15H16F3NO4
- The crystal structure of 1-phenylethan-1-aminium 4-hydroxy-3,5-dimethoxybenzoate C17H21NO5
- The crystal structure of 1,3,5-trichloro-2-nitrobenzene
- The crystal structure of tris(μ2-bromido)-bis(η6-p-cymene)-diosmium(II) tetrafluoroborate, C20H28BBr3F4Os2
- Crystal structure of new barium lithium manganese fluorides: Ba14Li1.87Mn14.13F68 with a Jarlite–related structure
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium chloride, C25H21ClFP
- The crystal structure of calcitriol–chloroform (1/1), C27H44O3⋅CHCl3
- The crystal structure of (E)-1-((3)-nitrophenyl)pyren-3-(pyren-1-yl)prop-2-en-1-one, C25H15NO3
- Crystal structure of (E)-2-hydroxy-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H16N2O3
- Crystal structure of (E)-(3-(thiophen-2-yl)acryloyl)ferrocene, C17H14FeOS
- Crystal structure of (E)-(3-(furan-2-yl)acryloyl)ferrocene, C17H14FeO2
- Synthesis and crystal structure poly[diaqua(μ3-3-(((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl) methyl)ammonio)propanoate-κ3 O:O′:O″) sodium(I)] monohydrate, C20H24NNaO12S
- Crystal structure of 9-methoxy-4-(2-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine C19H18N4O2
- Synthesis and crystal structure of 4-(difluoromethyl)-1-methyl-N-(pyridin-3-yl)-1H-pyrazole-3-carboxamide hydrate, C11H12F2N4O2
- The crystal structure of caesalfurfuric acid B, C22H32O4
- The crystal structure of 2-bromo-2-(5-bromo-2-methyl-4-nitro-1H-imidazol-1-yl)-1-phenylethanone, C12H9Br2N3O3
- The crystal structure of bis{chlorido-[μ2-(1-oxidopyridin-2-yl)(pyridin-2-yl)amido-κ3 O,N, N′]copper(II)}, C20H16Cl2Cu2N6O2
- The crystal structure of 3-amino-2-formyl-1-phenyl-9,10-dihydrophenanthrene-4-carbonitrile, C22H16N2O
- The crystal structure of 1,1′-(2,5-dimethylpyrazine-1,4-diyl)bis(ethan-1-one), C10H14N2O2
- Crystal structure of 5′-(9-phenyl-9H-carbazol-3-yl)-[2,2′-bithiophene]-5-carbaldehyde, C27H17NOS2
- The crystal structure of the double salt dipyridin-1-ium bromide tribromide
- Crystal structure of (E)-(3-(3-methylthiophen-2-yl)acryloyl)ferrocene, C18H16FeOS
- Crystal structure of (E)-(3-(4-phenoxyphenyl)acryloyl)ferrocene, C25H20FeO2
- Crystal structure of (E)-(3-(3,4-dimethylphenyl)acryloyl)ferrocene, C21H20FeO
- Crystal structure of [(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N‴)tetracyanidodiplatinum(II)] dimethyl sulfoxide solvate, C18H36N8O2Pt2S2
- Crystal structure of (4-ethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO2P
- Crystal structure of (1-naphthalen-1-yl-methyl)triphenylphosphonium chloride ethanol solvate, C31H30ClOP
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)platinum(II) bis[tribromido(dimethyl sulfoxide-κS)platinate(II)], C14H36Br6N4O2Pt3S2
- Crystal structure of (2-methylbenzyl)triphenylphosphonium chloride ethanol solvate, C28H30ClOP
- Crystal structure of bis(η2, σ1-8-methoxycyclooct-4-enyl)(μ2-1,4,8,11-tetraazacyclotetradecane-κ4 N, N′, N″, N‴)diplatinum(II) dibromide, C28H54Br2N4O2Pt2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)palladium(II) tetrabromidopalladate(II), C10H24Br4N4Pd2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)palladium(II) bis[trichlorido(dimethyl sulfoxide-κS)platinate(II)], C14H36Cl6N4O2PdPt2S2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)palladium(II) tetraiodidopalladate(II), C10H24I4N4Pd2

